
It's really difficult for all of us to see when we are being programmed or manipulated through advertisement/propaganda. But pharmaceutical companies spend billions of dollars on conditioning the population to choose its products over other methods of treatment. And sometimes we’re in an automatic mode when we decide our choice in healthcare. This can be a problem for a number of reasons. The first thing to consider is if you are deciding to utilize a drug as your first option you might not understand the dangers of that approach.

Besides the over 2000 deaths per week due to the side effects of pharmaceutical drugs and the fact that medical care is a number one cause for death in the United States based on CDC statistics, there are other reasons to think twice about drugs being your first approach. Simply put drugs don't heal, drugs alter your perception of the condition. Let's take headaches, for example, most headaches are caused by misalignments in the upper neck (muscle tension) other causes could be toxicity problems, allergies, low blood sugar or hormonal problems. Which of these do you think an aspirin fixes? Did you know aspirin causes bleeding in the stomach, Excess Stomach Acid Secretion, Stomach Cramps, Blood coming from Anus, Decreased White Blood Cells? This is just a partial list of potential side effects. Again the aspirin doesn't treat any of the underlying causes for your condition.
Let's say your headaches were related to hormonal imbalances because of a developing ovarian cyst. You keep treating the headaches with an aspirin and your ovarian cyst keeps getting worse to the point that it might burst. Wouldn't it have been nice if the physician you went to wanted to understand the source of your headaches? But a common problem is if you're a physician and have powerful drugs that can mask symptoms then when a patient presents with certain symptoms you are just gonna write a prescription and consider it appropriate care.
As a chiropractic physician, we don't mask symptoms with drugs, we have to figure out why the symptom is there. Just to classify a headache is a tension headache or a migraine headache and then give the latest masking drug the pharmaceutical company has recommended, in my opinion, is not proper healthcare. Proper healthcare tries to determine the underlying cause for the condition and correct the cause. I'm not saying that can be done 100% of the time because science does not understand the body fully, I am saying that most the time it can be. The underlying cause of allergies is not an antihistamine deficiency it's an inappropriate functioning immune system. When you correct the immune system you correct the allergies. Acid reflux is not an antacid deficiency it is an imbalance somewhere within the digestive system. It needs to be found and corrected which might include changing your diet.
Back pain is not a muscle relaxer or anti-inflammatory deficiency disease. When you just treat the symptoms you allow the condition time to get worse. This is one of the reasons that chronic diseases are such a high prevalence in our society. When you chronically just treat symptoms over a lifetime you end up with a basket full of chronic conditions. And this is why many people live their last 10 or 15 years of life in a nursing home or a wheelchair suffering from multiple health problems. If you don't want to end up there you have to change the choices you make with your body. The fact is most drug therapy is inappropriate. Let me make this clear there is a time for drugs and there is a time for surgeries but they should be the last approach. Unfortunately, when you let certain conditions develop to the point of no return you eliminate a number of your options to get well.
Natural healthcare focuses on health, not disease. Disease care has led to horrible health statistics for countries that focus their attention there. When a type II diabetic is taking one, two or three different medications to try to control their blood sugar they are in fact shortening their lifespan and making chronic disease more likely. The sad thing is the vast majority of type II diabetics can be drug-free and healthy by some simple dietary and nutritional approaches. Lowering your blood sugar with drugs to force the sugar out of the body is not the same as correcting the blood sugar problem. Find the cause, correct the cause.

It is true that antibiotics can help clear acne in patients who suffer from it but it's also true that antibiotics will wreck havoc on your digestive tract and weaken your immune system. Is acne an antibiotic deficiency disease? The skin is just the expression of the health of the body. Acne has to do with body ecology, hormonal balance and sometimes food allergies. The chances are the use of antibiotics will make each of these issues worse. And has anybody heard of superbug infections? The overuse of antibiotics is the number one cause of death from superbug infections that have been growing every year for the past 25 years. Is there a place for antibiotics? There is but it's very rare. There are so many natural immune boosting techniques in natural healthcare that well over 90% of my patients never require any antibiotics in their life as long as they are following the approaches we recommend. Sure there are exceptions to every rule but in our society, the exception is the rule.

Do you want to be healthy? Drugs are not going to get you there. Drugs could save your life but the same drugs that saved your life could end your life if you keep taking them. Drugs are toxic, drugs are dangerous. If you want to be healthy then you have to practice a healthy lifestyle. Did you know your nervous system controlled and coordinated everything that happens in your body? That's why we focus on spinal health to maintain a proper nervous system function. That is one of the five laws of health that we encourage patients to follow. The second law would be the law of diet. Do you really believe you could eat Twinkies all day and stay healthy? Of course not. But just like the big Pharma, big food has programmed you into digging your own grave with your fork. You have to eat a diet that's consistent with your genetic makeup and actually has real food in it. Eating right is really not that hard with a little direction you can increase your energy levels, strengthen your cardiovascular systems and maintain proper blood sugar levels very easily. The third law is exercise. You don't have to run marathons, in fact, you can over-exercise to your detriment. Exercise is also related to your genetic makeup. Some people doing the wrong type of exercise can actually hurt themselves. People who have some type of moderate, regular exercise will be healthier than those who don't. Law four is a positive mental and spiritual attitude. Life can be hard you need to have coping skills in order to be successful. Spiritual disciplines have been shown by research to make us healthier and to allow us to live a longer life. In the fifth law is rest and relaxation.
None of these laws are really difficult to follow you just need to find yourself in a culture that encourages these. The pharmaceutical approach is not that culture. As nice as it is to have a fire department it's also a lot better if you never have to call them. And in most circumstances with a little prudence, those disasters can be avoided. One of the best ways to find yourself in the culture of better health is to choose natural healthcare. Making natural healthcare your first choice can prevent a number of disasters in your life and your families health. You still might be referred to a medical specialist from time to time but if your primary care is a physician in the natural healthcare field your outcomes would be greatly improved.

Are you locked in the matrix? Choose natural healthcare and choose a better life.
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111
IN THE NEWS:. STD Prevalence Skyrockets Amid Concerns Over Growing Antibiotic-Resistance Three of the more commonly transmitted diseases have reached record levels in the U.S. Nationwide, there were 1.6 million cases of chlamydia in 2016, 470,000 cases of gonorrhea and 28,000 new cases of syphilis. Mutations of the Neisseria gonorrhoeae bacteria that cause gonorrheal infections have led to a high incidence of antibiotic resistance, making it extremely difficult to treat. Research looking at syphilis samples from the U.S., South America, Europe, Africa and Australasia found both of the two main strains of syphilis have developed antibiotic resistance. STD prevalence in California has increased by 45 percent in the past five years. In 2017, 300,000 cases of chlamydia, gonorrhea and syphilis were reported, 54 percent of cases occurring in those under the age of 25. The number of babies born infected with syphilis quadrupled, and with it, stillbirths spiked as well. Of the 278 congenital syphilis cases on record in California last year, 30 resulted in stillbirth, which is triple the number of syphilis-related stillbirths reported in 2016.

WELLNESS: Mercury poisoning: Ways “silver” dental fillings can destroy your health Many integrative healthcare providers describe chronic exposure to mercury as a “biochemical train wreck” and an “underreported epidemic.” To find the primary source of low-level mercury poisoning, many of us need look no further than our own dental work. “Silver” dental fillings, used on almost half of all dental restorations in the United States, contain 45 to 55 percent mercury – and the simple acts of chewing and drinking hot liquids can cause the release of harmful mercury vapors. Accumulating in the brain, kidneys, liver, lungs and gastrointestinal tract, mercury jeopardizes human health in literally dozens of ways. The symptoms of mercury poisoning listed below range in severity from merely annoying to potentially life threatening. But be advised: as shocking as it seems, this overwhelming litany of harm is just a partial list. Emotional and psychological symptoms According to the IAOMT, mercury exposure can cause a “baker’s dozen” (more or less) of truly disturbing psychological symptoms – including aggression, suicidal ideation, fits of rage, mood swings and manic depression. Gastrointestinal problems Mercury disrupts the intestinal flora of the gut microbiome, the community of beneficial bacteria that helps to regulate health. This can result in digestive complaints such as diarrhea, constipation, loss of appetite and weight loss. Other GI symptoms from low-level mercury poisoning include nausea, vomiting and heartburn. Respiratory difficulties asthma, allergies, bronchitis, emphysema, chest congestion, shortness of breath and shallow respiration. Reproductive issues Reproductive problems linked to mercury include Infertility, lowered sperm count, premenstrual syndrome, genital discharge and spontaneous abortions. Neurological disorders Tremors, paralysis, neuropathy, epilepsy, headaches, dizziness and vertigo have been reported with mercury poisoning. Impaired immune system According to Dr. McGuire, mercury poisoning can significantly impair the immune system, raising susceptibility to disease and causing autoimmune disorders.
CHIROPRACTIC: Chiropractic is a healthcare discipline that emphasizes the inherent recuperative power of the body to heal itself without the use of drugs or surgery. The practice of chiropractic focuses on the relationship between structure (primarily the spine) and function (as coordinated by the nervous system) and how that relationship affects the preservation and restoration of health. In addition, doctors of chiropractic recognize the value and responsibility of working in cooperation with other health care practitioners when in the best interest of the patient. One of the best things about receiving chiropractic adjustments is that they are a completely drug-free path to healing the body naturally. Chiropractic benefits including helping to naturally improve problems such as: Back pain, Headaches, Bowel regularity, Improved mental clarity, Ear infections, Neck pain, Arthritis and joint pain, Scoliosis, Asthma, Blood pressure, Healthy pregnancy, Organ function, Surgery prevention. Despite its popularity, there are still a lot of misconceptions about the field of chiropractic care, including how the practice works and how chiropractors are trained. For example, did you know that many chiropractic programs incorporate an entire year of PhD-level advanced nutrition training? Think chiropractic first, drug second and surgery last.
FUNNY BONE: Thank God I don't have to hunt for my food, I don't even know where tacos live...@@That frail moment when you pull your blankets up and punch yourself in the face.@@When people tell me "you're going to regret that in the morning," I sleep in until noon because I'm a problem solver.@@It's okay if you disagree with me, I can't force you to be right.@@Dear pimples if you're going to live on my face, I need to see some rent.@@Working in a mirror factory is something I can totally see myself doing.@@Someone stole my Microsoft Office I said, “ they're gonna pay. You have my Word.”@@I feel bad for the homeless guy, but I feel really bad for the homeless guy’s dog, It must be thinking "man, this is the longest walk ever."@@Someone stole my mood ring, I don't know how I feel about that.
Visit our web site: keefeclinic.com-faceebook/keefeclinic.com
In the West, traditional eastern medicines are not usually recognized until there is scientific evidence to back it up. Well, in the case of turmeric, there is!

(1) Kills 16-times more cancer cells than the leading chemo drug Eloxatin — without harming healthy cells (International Journal of Oncology)
(2) Performs better in memory tests than the drug Aricept (the most widely prescribed Alzheimer's drug) — Salk Institute for Biological Studies
(3) Lowers cholesterol and triglyceride levels better than the statin drug Lipitor (Journal of Drug Research and Development)
(4) Beats Celebrex for relieving knee arthritis pain (Journal of Alternative and Complementary Medicine)
(5) Relieves rheumatoid arthritis pain better than Ibuprofen (Journal of Phytotherapy Research)
(6) “Therapeutic effects are comparable to pharmaceutical NSAIDs... but with a major difference in that this compound is nontoxic and free of side effects.” - Vanderbilt and University of Pittsburgh researchers (Journal of Surgical Neurology International)
(7) “More effective in stopping the protein fragments from forming than many other drugs being tested to treat Alzheimer's” - UCLA Alzheimer's Department and Veterans Affairs researchers (Journal of Biological Chemistry)
(8) “It's 400-times more potent than the diabetes drug Metformin” — reports Auburn University researchers (Journal of Biochemical and Biophysical Research Communications)
(9) More effectively treats Major Depressive Disorder (MDD) than Prozac — without Prozac's devastating side effects, according to a randomized, controlled study.
(10) Treats chronic uveitis — a leading cause of blindness — better than corticosteroids... the only available prescription treatment (Journal of Phytotherapy Research)
(11) “Could enhance erectile function with more efficacy and more prolonged duration of action than Viagra” (International Journal of Impotence Research)
(12) Destroys more colon cancer stem cells than FOLFOX (one of the most widely prescribed chemotherapy protocols) - Baylor University researchers
http://www.mdpi.com/2072-6643/10/5/604/htm
Open Access
Review
Inflammation, not Cholesterol, Is a Cause of Chronic Disease
Alexandros Tsoupras, Ronan Lordan and Ioannis Zabetakis *
Department of Biological Sciences, University of Limerick, V94 T9PX Limerick, Ireland
Received: 23 April 2018 / Accepted: 9 May 2018 / Published: 12 May 2018
Abstract
:
Since the Seven Countries Study, dietary cholesterol and the levels of serum cholesterol in relation to the development of chronic diseases have been somewhat demonised. However, the principles of the Mediterranean diet and relevant data linked to the examples of people living in the five blue zones demonstrate that the key to longevity and the prevention of chronic disease development is not the reduction of dietary or serum cholesterol but the control of systemic inflammation. In this review, we present all the relevant data that supports the view that it is inflammation induced by several factors, such as platelet-activating factor (PAF), that leads to the onset of cardiovascular diseases (CVD) rather than serum cholesterol. The key to reducing the incidence of CVD is to control the activities of PAF and other inflammatory mediators via diet, exercise, and healthy lifestyle choices. The relevant studies and data supporting these views are discussed in this review.
Keywords:
cardiovascular disease; atherosclerosis; inflammation; platelet-activating factor; oxidised lipoproteins; cholesterol; chronic diseases
1. Introduction
1.1. Biological Significance of Cholesterol—Circulating Blood Cholesterol
Cholesterol, an unsaturated alcohol of the steroid family, is essential for the normal function of all animal cells. It is also a fundamental element for the normal structural makeup and the fluidity of all cell membranes. Cholesterol interacts with phospholipid bilayers in the cell membrane and increases membrane packing. Cholesterol also takes part in signal transduction, intracellular transport, nerve conduction, and signalling pathways through lipid rafts and caveolae. Cholesterol has various other biological functions, i.e., it is a precursor molecule for several biochemical pathways such as the synthesis of vitamin D, steroid hormones (e.g., cortisol, aldosterone, and adrenal androgens), and sex hormones (e.g., testosterone, oestrogens, and progesterone). Cholesterol is also a constituent of bile salts, which are crucial constituents of digestion, as they facilitate the absorption of lipids, fats, and fat-soluble vitamins A, D, E, and K [
1].
Since cholesterol is mostly a lipophilic molecule, it does not dissolve well in blood. For this reason, it is packed into lipoproteins that are composed of a lipid core (which can contain cholesterol esters and triglycerides) and a hydrophilic outer membrane comprising phospholipids, apolipoprotein, and free cholesterol. This allows for the transport of the nonpolar lipid molecules such as cholesterol and triglycerides around the body through the blood to cells that require them. Plasma lipoproteins are separated into five major classes: chylomicrons, very-low-density lipoproteins (VLDL), intermediate-density lipoproteins (IDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) [
1,
2].
Cholesterol can enter the blood through the digestion of dietary fat via chylomicrons. However, since cholesterol has an important role in cellular function, it can also be directly synthesised by each cell in the body. Notably, LDL particles are thought to act as a major transporter of cholesterol to the peripheral tissues, as at least two-thirds of circulating cholesterol resides in LDL. Conversely, HDL molecules are thought to do the opposite. They take excess cholesterol and return it to the liver for excretion [
1,
2].
Recent evidence suggests that dietary intake of cholesterol can influence plasma and serum levels, but not significantly. However, this is still subject to debate and further study [
3]. Plasma cholesterol levels along with the levels of LDL cholesterol, HDL cholesterol, and serum triglycerides are currently used as biomarkers of the so-called standard ‘lipid profile’ for each individual. The standard lipid profile has been widely used as a traditional biomarker, not only for cardiovascular health but also for other lipid-related abnormalities and disorders [
4].
1.2. Cholesterol Levels: Demonising a Risk Factor but Not the Causative Mechanisms of Chronic Diseases
Several modifiable and non-modifiable risk factors (genetic, environmental, nutrition, and lifestyle, etc.) are thought to influence the balance between health and disease by inducing mechanisms related to disease onset, development, and the manifestations of symptoms. The presence or coexistence of these risk factors seem to trigger underlying molecular and cellular mechanistic pathways that can lead to continuous chronic manifestations and the long-term loss of tissue homoeostasis and tissue dysfunction. These continuous chronic manifestations can develop over time before cellular disturbances manifest and cause tissue disorders, while, if not counterbalanced by our immune system and by specific preventive measures such as a healthy diet and lifestyle, the subsequent symptomatic disease finally appears, and medical treatment may be required to reduce the risk of mortality. Elucidating these molecular and cellular mechanistic pathways and acquiring the mechanistic evidence of the underlying multifactorial causes of a chronic disease can lead to suitable preventive targets against these diseases with fewer side effects, which is an ongoing difficult and demanding task. Such difficulties have misled the scientific and medical community to often and lightly extrapolate the easily acquired observed statistical and epidemiological correlations of traditional risk factors to several chronic diseases, towards matching these risk factors as the causative agents of these diseases.
According to the ‘cholesterol hypothesis’, high blood cholesterol is a major risk factor, while lowering cholesterol levels can reduce risk [
5]. Dyslipidaemias (i.e., hypercholesterolaemia or hyperlipidaemia) are abnormalities of lipid metabolism characterised by increased circulating levels of serum total cholesterol, LDL cholesterol, triglycerides, and decreased levels of serum HDL cholesterol. High levels of LDL cholesterol and non-HDL cholesterol have been associated with cardiovascular risk, while other cholesterol-related serum markers, such as the small dense LDL cholesterol, lipoprotein(a), and HDL particle measurements, have been proposed as additional significant biomarkers for CVD risk factors to add to the standard lipid profile [
6]. HDL cholesterol has been considered as the atheroprotective ‘good’ cholesterol because of its strong inverse correlation with the progression of CVD [
7]; however, it is the functionality of HDL cholesterol, rather than its concentration that is more important for the preventative qualities of HDL cholesterol in CVD. In general, dyslipidaemias have been ranked as significant modifiable risk factors contributing to prevalence and severity of several chronic diseases including aging, hypertension, diabetes, and CVD. High serum levels of these lipids have been associated with an increased risk of developing atherosclerosis [
8].
Furthermore, dyslipidaemias have been characterised by several studies not only as a risk factor but as a “well-established and prominent cause” of cardiovascular morbidity and mortality worldwide [
9]. Even though such an extrapolation is not adequate, it was, however, not surprising that this was made, because since the term arteriosclerosis was first introduced by pioneering pathologists of the 19th century, it has long been believed that atherosclerosis merely involved the passive accumulation of cholesterol into the arterial walls for the formation of foam cells. This process was considered the hallmark of atherosclerotic lesions and subsequent CVD. Moreover, one-sided interpretations of several epidemiological studies, such as the Seven Countries Study (SCS), have highlighted outcomes that mostly concerned correlations between saturated fat intake, fasting blood cholesterol concentrations, and coronary heart disease mortality [
10,
11,
12,
13]. Such epidemiological correlations between dyslipidaemias and atherosclerosis led to the characterisation of atherosclerosis as primarily a lipid disorder, and the “lipid hypothesis” was formed, which would dominate thinking for much of the 20th century.
In the clinical setting, in order to address the lipid hypothesis, the levels of cholesterol related plasma lipoproteins and triglycerides (lipid profile) have been used as traditional biomarkers for cardiovascular risk, but also for dietary and treatment guideline designs [
5]. Dietary and medical guidelines have focused on the reduction of cholesterol and lipid levels as the best way to prevent chronic diseases such as CVD [
5,
9]. Such guidelines suggest the application of statin therapies in order to reduce the levels of cholesterol (through inhibition of cholesterol synthesis by HMG-CoA reductase inhibitors); however, numerous side effects have been reported, including the development of other chronic diseases such as diabetes mellitus [
14]. Moreover, specific dietary strategies for reducing cholesterol intake are the mainstay of management in most cases of dyslipidaemia, prior to, or simultaneously with, the initiation of a lipid lowering agent [
9]. Dietary fats, cholesterol, and the levels of serum cholesterol in relation to the development of CVD have been somewhat demonised.
On the other hand, since cholesterol is an essential biomolecule for the normal function of all our cells, an emerging question has recently surfaced: “how much do we need to lower the levels of cholesterol”? Furthermore, given the fact that cholesterol plays a crucial role in several of our cellular and tissue mechanisms, it is not surprising that there are several consequences due to the aggressive reduction of cholesterol levels in the body, which has been common practice over the last few decades. In addition, targeting cholesterol and fat intake by introducing diets with low-fat products and by reducing the intake of high-fat foods can lead to less absorption and lower bioavailability of other lipids containing high value nutrients, such as several lipid soluble vitamins (especially vitamin D) and other lipid molecules. Such lipids have exhibited a plethora of beneficial bioactivities, not only related to reducing the risk of chronic diseases but also through a wide range of important bio-functionalities and anti-inflammatory properties [
3]. Therefore, lower cholesterol levels do not equate to better health, or to lower risk of chronic diseases such as CVD. Homeostasis must be maintained, even with regard to cholesterol, both HDL and LDL [
15].
Moreover, recent systematic reviews and meta-analyses have started to question the validity of the lipid hypothesis, as there is lack of an association or an inverse association between LDL cholesterol and both all-cause and CVD mortality in the elderly [
15] and several cancers such as lung, prostate, and breast cancer [
16,
17,
18]. Such studies provide the rationale for more research about the causes (and not only the risk factors) of chronic diseases such as atherosclerosis, CVD, and cancer, but also for a re-evaluation of the guidelines for cardiovascular prevention, in particular because the benefits of statin treatments have been exaggerated [
15].
Statistical and epidemiological extrapolations often lack fully clarified biochemical mechanistic evidence, while associations and correlations do not necessarily mean causation. In addition, a follow-up by systematic reviews and meta-analyses often present contradictory outcomes against the initial results that were introduced by early stage epidemiological studies lacking consistency, biological gradient, and coherence. Thus, such extrapolations can lead to one-sided, premature targeting of risk factors accompanied with consequences, often without the desirable outcomes. Targeting a risk factor such as high serum cholesterol may decrease the probabilities for a disease, but usually cannot prevent the causation of chronic diseases.
1.3. Revisiting the Lipid Hypothesis: Outcomes of the Mediterranean Diet against Inflammation
Previous epidemiological and observational studies, such as the SCS in which the lipid hypothesis was mostly based, have been re-evaluated. For example, even though within the SCS the strength of the association between serum cholesterol and cardiovascular mortality were similar in different cultures, the absolute risks differed substantially. Kromhout reported that at a serum cholesterol level of 200 mg/dL, the 25-year cardiovascular mortality rate was five times higher in the Northern European populations of the SCS compared to the Southern Mediterranean populations [
19], and thus the relations between diet, serum cholesterol, and cardiovascular mortality are more complex than originally thought. This is because it is not only dietary cholesterol involved, but other lipids and antioxidants may play a role in the onset and prevention of atherosclerosis [
19]. Such a low prevalence of cardiovascular mortality in the Mediterranean cohorts of SCS is now attributed to their lifestyle and especially to their dietary habits, namely the traditional Mediterranean diet (Med-diet) [
10,
20]. A common feature of the diet amongst populations in the Mediterranean is a relatively high dietary intake of vegetables, fruits, legumes, whole grains, monounsaturated fats, and nuts, followed by moderate consumption of fish, dairy products (mainly cheese and yogurt), alcohol, and low consumption of red and processed meats [
21].
The major outcomes of the SCS and other similar epidemiological studies (i.e., studies trying to decipher the ‘French Paradox’ [
22]) concerning the protective effects of dietary patterns, such as the Med-diet against chronic diseases, were initially either neglected or misinterpreted. CVD and cardiovascular mortality occurred in much relatively lower rates in the Southern European populations (i.e., Italy and Greece) despite a rather high dietary intake of saturated fats and cholesterol [
10,
20,
23]. A recent systematic review and meta-analysis revealed that Med-diet can actually reduce the incidence of cardiovascular events, breast cancer, and type II diabetes mellitus, without any restriction on fat intake [
24].
Over the last 2 years there has been a significant number of studies referring to adoption of the Med-diet pattern and its associated beneficial outcomes in a plethora of several chronic diseases that are either directly or indirectly related to inflammation. These studies refer to heart failure, CVD [
25], cancer [
26,
27], obesity [
28], metabolic syndrome [
29,
30,
31], diabetes [
31,
32,
33,
34], and other subsequent manifestations such as diabetic retinopathy [
35], asthma [
36], autoimmune diseases such as rheumatoid arthritis [
37], incident frailty risk [
38], non-alcoholic fatty liver disease [
39,
40], inflammatory bowel disease [
41], cognitive health, the risk of Alzheimer’s disease and dementia [
42,
43,
44], and age-related macular degeneration [
45].
In addition, the Med-diet has also been associated with beneficial outcomes, even in secondary CVD prevention [
46]. When patients suffering from CVD or diabetes follow the Mediterranean dietary pattern, the incidence of recurrent myocardial infarction and cerebrovascular events is reduced. The protective effect of this dietary pattern can be maintained for up to four years after the first infarction (Lyon Diet Heart Study) [
47]. Moreover, in contrast to the contradictions of lipid hypothesis and mortality in elderly people [
15], the HALE project has also shown that individuals aged 70 to 90 years following a Med-diet and healthy lifestyle have a 50% lower rate of all-cause and cause-specific mortality [
48]. Followers of the Med-diet are also less likely to suffer sudden cardiac death and age-related cognitive decline [
49].
The inverse association between Med-diet and all causes of diseases and cardiovascular-mortality has been attributed to several of its pleiotropic protective effects. For instance, the Med-diet can beneficially influence several risk factors such as lowering BMI, blood pressure, reducing insulin resistance, reducing lipid levels (i.e., the ratio of cholesterol/HDL cholesterol), and improving HDL-cholesterol functionality [
50,
51,
52,
53,
54]. However, the main beneficial impact of Med-diet is on the improvement of endothelial function and the decrease of the inflammatory milieu, inflammation-related mediators, biomarkers such as platelet-activating factor (PAF), and several cytokines. It is also suggested that there is an improvement of oxidative stress, with lower concentrations of oxidised LDL and improved apolipoprotein profiles, and, finally, there is evidence of beneficial effects against platelet aggregation and blood coagulation [
3,
55,
56,
57,
58].
The overall outcomes and beneficial effects of Med-diet have radically shifted the attention from the lipid-centric model that is characterised by the desired reduction of cholesterol levels to more effective targeting against the factual causative factors of chronic diseases, which are inflammation and its related manifestations. Prevention is key to reducing global mortality due to chronic diseases such as CVD; therefore, it is imperative to separate the underlying causes and processes of the disease from the risk factors and symptoms of disease. The clarification of the key roles and interplay of various cells, inflammatory mediators, and pathways during chronic inflammatory manifestations related to the onset of several chronic diseases is of great importance and may lead to a plethora of novel potential targets for fine-tuning of the inflammatory response during the chronic smouldering of inflammation that characterises these disorders.
2. Re-Discovering Chronic Inflammation as the Cause for Chronic Diseases
Inflammation is a physiological reaction of the innate immune system that maintains a constant internal milieu while being exposed to continuously changing environmental pressures, irrespective of whether the initial causes originate from mechanical, physical, chemical, infectious, immunological, or reactive natural traumatic injury or metabolic dysfunction. The inflammatory response aims to reduce the agent that causes tissue injury and/or minimise these effects, to induce appropriate wound healing and to restore tissue homeostasis. Inflammatory responses are initiated by innate sensing mechanisms that detect the presence of microbial infection, stressed or dying cells, loss of cellular integrity, barrier breach, etc. A cascade of inflammatory pathways and mechanistic effects is supposedly well-orchestrated by the immune system in order to eradicate the causative agent.
Several immune cells can change their number, morphology, and nature depending on the stage and type of inflammation. Biochemically, inflammation is denoted by a local increase of numerous tissue hormones, transmitters, complement components, cytokines, and lipid mediators such as PAF and eicosanoids. Most of these products are autacoids that are synthesised at the site of inflammation in order to resolve the inflammatory process by removing or inhibiting the actions of the triggering agent [
8]. Provided that the immune response succeeds in eliminating the infectious agent or to repair the initial tissue injury, the inflammatory process will be terminated in a timely fashion and thus only affects tissue function transiently.
However, in cases where the inflammation fails to resolve due to the persistence of the triggering agent or due to unsuccessful repair of the initial tissue injury or dysfunction, a sustained underlying inflammatory process develops, leading to further tissue dysfunction and detrimental consequences. Several traditional and emerging risk factors are thought to influence our health and, especially, inflammation-related chronic diseases, by their interrelation with underlying molecular and cellular manifestations that result in chronic inflammatory responses leading to the loss of tissue homoeostasis and dysfunction. Apart from dyslipidaemias, other well-established risk factors include hypertension, diabetes, smoking, excessive food intake, previous infections (influenza, oral pathogens) or underlying autoimmune diseases such as lupus or rheumatoid arthritis, pollution, and genetic abnormalities [
59]. It is now well established that a common junction of such risk factors is chronic and unresolved inflammatory manifestations. Inflammation that causes endothelial dysfunction seems to be the key causative underlying mechanistic player, at the molecular and cellular level, for the onset and development of subsequent inflammation-related chronic disorders such as atherosclerosis and subsequent CVD, ischemic and renal disorders, cancer metastasis, diabetes, infections, and comorbidities [
8,
57,
58,
59,
60,
61,
62].
For example, in cases of dyslipidaemia, increased cholesterol levels are not the causative agent or the underlying biochemical mechanism responsible for endothelial dysfunction and atherosclerosis development. The accumulation of excess plasma LDL cholesterol is addressed by the innate immune system as an undesired event. Therefore, an inflammatory response at the endothelial wall is promoted to reduce the threat by the removal of excess LDL and oxidised-LDL (Ox-LDL) cholesterol from the blood stream to the subendothelium, where they are engulfed by comigrated monocytes for final removal [
63,
64]. During chronic inflammatory diseases, inflammation and infections can also induce a variety of alterations in lipid metabolism, including decreases in serum HDL cholesterol, increases in triglycerides, lipoprotein(a), and LDL levels. These changes of the lipid levels may initially dampen inflammation or fight infection; however, the sustained inflammation can contribute to the increased risk of atherosclerosis [
65]. In addition to affecting serum lipid levels, inflammation also adversely effects lipoprotein function; LDL is more easily oxidised, as the ability of HDL to prevent the oxidation of LDL is diminished, while several steps in the reverse cholesterol transport pathway are also adversely affected during inflammation. The greater the severity of the underlying inflammatory disease, the more consistently these abnormalities in lipids and lipoproteins are observed [
65]. Thus, it is not serum cholesterol and lipoproteins that influence the endothelium but the inflammatory response that affects the well integrity and functionality of the endothelium.
Apart from the effects of inflammation on plasma lipids, it is now well established that more important soluble and cellular immune factors associated with chronic inflammation can promote inflammation-related endothelial dysfunction and atherogenesis, either during dyslipidaemia or independently of dyslipidaemia [
66]. Even though atherosclerosis and CVD were previously viewed as lipid storage disorders, we now recognise that inflammation drives much of endothelial dysfunction and mechanisms of clinical complications with these diseases and related comorbidities, such as sepsis [
67,
68], human immunodeficiency virus (HIV) infection [
69,
70,
71,
72,
73,
74], periodontal diseases [
75,
76,
77], kidney disorders [
78,
79,
80,
81], healthy ageing, and inflammatory autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis, independently of traditional cardiovascular risk factors such as serum lipid levels [
66,
82,
83].
Inflammation plays a key role in all stages of the formation of vascular lesions maintained and exacerbated by several risk factors such as unhealthy diet and lifestyle, smoking, hyperlipidaemia/hypercholesterolaemia, hypertension, autoimmune diseases, etc. The consequence of chronic inflammation is endothelial dysfunction that sets in, and we can define it as an integrated marker of the damage to arterial walls by classic risk factors. Endothelial dysfunction is usually characterised by an inflammation-related milieu acting on leukocytes and endothelial cells, through an interplay with other immune cells such as T lymphocytes, mast cells, dendritic cells (DC), and platelets [
57,
58,
66,
84,
85]. The orchestrated overexpression and increased production of pro-inflammatory cytokines occurs, including interleukin-6 (IL-6), tumour necrosis factor (TNF) and its receptor, high-sensitivity C-reactive protein (hsCRP), type I interferons (IFN-α, IFN-β), adhesion molecules, chemokines, and lipid inflammatory mediators such as PAF and eicosanoids. Other linked events include the increased generation of reactive oxygen species (ROS), the increased oxidation of LDL cholesterol, and the reduction of protective nitric oxide levels.
Therefore, the mechanistic pathways and key players implicated in the inflammatory crosstalk taking place throughout the onset, development, and progression of chronic diseases is of great importance, in order to unravel putative preventive and therapeutic targets with less side effects. The inverse effects of the Med-diet with chronic diseases is mostly related to the pleiotropic effects and interplay of its food constituents on all these inflammation-related pathways; following a Mediterranean dietary pattern leads to the reduction of several inflammatory mediators and biomarkers related to the endothelial functionality, such as decreases in hsCRP, IL-6, and intracellular adhesion molecule-1 (ICAM-1) [
27].
3. The Role of PAF in Chronic Diseases and the Beneficial Effects of the Mediterranean Diet
3.1. PAF Structure, Activities, and Metabolism: The Role of PAF
3.1.1. PAF Structure and Physiological Roles
PAF is a potent lipid inflammatory mediator with pleiotropic effects that are implicated in several chronic diseases [
57]. The classic PAF molecule is characterised by an alkyl ether linkage at the
sn-1 position, an acetyl group at the
sn-2 position, and a phosphocholine group at the
sn-3 position of glycerol backbone (1-O-alkyl-2-
sn-acetyl-glycero-3-phosphocholine, [
86]) (A). These three structural features are all equally important requisites for the optimal biological activity of PAF, mediated by its stereospecific binding to its specific receptor [
87,
88]. Because of the ether linkage at the
sn-1 position, the classic molecule of PAF is an unusual lipid, as such moieties are not common in animals, nor is it common to find the acetic acid esterified directly to glycerol at the
sn-2 position. Thus, it seems that PAF was chosen by evolution to participate in specific functions in several of our cells, tissues, organs, and throughout the body. PAF was the first intact phospholipid known to have messenger functions by binding to a specific receptor on the cell membrane, and not simply via physicochemical effects on the plasma membrane of the target cell [
79].
Figure 1. (
A) Typical structure of classic platelet-activating factor (PAF) molecule [
86]. (
B) Representative structures of bioactive polar lipids (PL) towards the PAF inflammatory pathways (
B), which have been identified in several foods of the Mediterranean diet [
56].
Lately, the term ‘PAF family’ has been proposed to include every other phospholipid molecule called PAF-like molecules, which have similar structures to those of the classic PAF molecule, and they exhibit similar bioactivities [
89]. However, such PAF-like moieties are usually less potent than PAF by several orders of magnitude, i.e., increasing the chain length beyond 3 carbons at the
sn-2 position decreases its biological potency; likewise, altering the polar group at
sn-3 position decreases the potency of the molecule. The molecular composition of PAF varies depending on different species and cell types. Related PAF-like lipids include, for example, the acyl-phosphatidylcholine-PAFs (with a short chain acyl group at the
sn-2 position), ethanolamine-PAFs, inositol-PAFs, oxidised alkyl-acyl phosphatidyl glycerophosphocholines [
90,
91], and hydroxyl-alkyl acyl phospholipids [
76,
77].
PAF, in general, play a vital role in various physiological processes such as mediation of normal inflammatory responses, regulation of blood circulation and pressure, regulation of coagulation responses, glycogen degradation, brain function, reproduction, foetal implantation, lung maturation, initiation of parturition, and exocrine gland functions [
92]. However, PAF can be regarded as both a friend, since it is presumed to have evolved as part of a protective mechanism in the innate host defence system, but also as a foe, because of its involvement in uncontrolled inflammation-related pathological conditions [
93]. When present in excess, PAF has been implicated in the pathogenesis of several inflammation-related chronic disorders [
57]; thus, its synthesis, distribution, and degradation are all strictly controlled, as would be predictable for such a potent molecule with a wide range of diverse actions.
3.1.2. The PAF/PAF-Receptor Signalling Pathways
PAF and PAF-like molecules act through their binding to a unique G-protein coupled seven transmembrane receptors, called the PAF-receptor (PAF-R) [
87,
88]. Species identity, differentiated by heterogeneity in linkage, degree of unsaturation, and carbon chain length of the alkyl or acyl chains at the
sn-1 and
sn-2 position, partially dictates signalling specificity by eliciting various signal transduction pathways following PAF-R activation [
94,
95]. The PAF-R is constitutively present on platelets, leukocytes, and endothelial cells, and further expression may be induced by appropriate stimuli. PAF-R is highly expressed by cells within the innate immune and cardiovascular systems [
96], pointing to a role for PAF and PAF-like molecules as pleiotropic communicators in plasma [
97].
Ligand binding (PAF and/or PAF-like molecules) to the PAF-R subsequently triggers multiple intracellular signalling pathways and gene-expressions, depending on the target cell and PAF levels (concentration) in blood or tissue [
87,
88,
89,
98] ( (A1–A3)). For example, activation of the PAF-R signalling initiates (through a Gq-linked mechanism) PLCβ-mediated hydrolysis of PIP2 to produce IP3 and DAG, leading to transient elevation of cytosolic Ca
2+ released from intracellular stores and activation of PKC. The rise in Ca
2+ also activates cPLA2α, leading to the release of arachidonic acid (AA) and lysophosphatides, which can serve as substrates for further synthesis of eicosanoids and PAF, respectively. In addition, signalling through Gi-linked PAF-R inhibits the conversion of ATP to cAMP by adenylate cyclase, thus preventing the activation of PKA and related signalling events.
Figure 2. Role of PAF, PAF-R, and its related pathways in the inflammatory cascades and in the pathogenesis of inflammation-related chronic disorders; increased PAF levels by pro-inflammatory stimuli and binding of PAF on its receptor, PAF-R, on the membranes of several cell types can lead to intracellular cascades and a PAF cycle-related amplification of the initial stimuli (
A) and in numerous cell responses according to each cell type (
B), which can lead to endothelial dysfunction and the onset and progression of inflammation-related chronic diseases. A1. Several risk factors and related upstream pro-inflammatory stimuli trigger formation of PAF and PAF-like molecules (i.e., oxidised phospholipids) and expression of PAF-R. A2. Binding of PAF/PAF-like molecules on PAF-R promote several inflammation-related intracellular pathways; activation of the PAF-R signalling initiates (through a Gq-linked mechanism) PLCβ-mediated hydrolysis of PIP
2 to produce IP
3 and DAG, leading to transient elevation of cytosolic Ca
2+ released from intracellular stores and activation of PKC. The rise in Ca
2+ also activates cPLA
2α, leading to the release of AA and lysophosphatides, which can serve as substrates for further synthesis of eicosanoids and PAF, respectively. Signalling through Gi-linked PAF-R inhibits the conversion of ATP to cAMP by adenylate cyclase, in this way preventing the activation of PKA and related anti-inflammatory signalling events. A3. Activation of the PAF/PAF-R intracellular pathways leads to the activation of cPLA
2 and PAF biosynthetic enzymes (LPCAT) for further formation of PAF and other lipid second messengers, thus creating a PAF cycle and further amplification of the initial inflammatory stimuli, while expression of genes involved in inflammatory manifestations (such as genes of several cytokines, integrins, selectins, metalloproteinase, several enzymes for eicosanoids, and ROS, etc.) is also induced. The pathways inducing the PAF-CPT-related synthesis of PAF are not fully elucidated. B. Increased PAF levels at the site of inflammation and ligand binding (PAF and/or oxidised phospholipids binding) on PAF-R can promote a broad spectrum of PAF effects depending on the cell type and tissue, which is achieved through the production and release of various downstream mediators, such as PAF itself and several other mediators of inflammation such as eicosanoids, cytokines (i.e., TNF-α, IL-1α, IL-6, IL-8, INF-γ, etc.), growth factors (i.e., VEGF, IGF, TGF), ROS, and RNS, but also through the expression of selectins and integrins (i.e., ICAM, VCAM, P-Selectin, E-Selectin) in the membranes of activated cells. Thus, increased downstream mediators, PAF levels, and the subsequent further activation of the PAF/PAF-R pathways promotes the activation and aggregation of platelets and leukocytes, activation of endothelial cells, leukocyte adherence, motility, chemotaxis, invasion, migration, and subsequent endothelial dysfunction, thus stimulating the onset and development of inflammation-related chronic diseases and disorders. C. Microconstituents of several foods of the Mediterranean diet have been found to beneficially inhibit the PAF/PAF-R pathways and PAF synthesis towards homeostatic re-equilibration of PAF levels and activities [
57]. PAF: platelet-activating factor; PAF-R: G-protein-coupled PAF-receptor; AC: adenylate cyclase; NF-kB: nuclear factor-kappa light-chain-enhancer of activated B cells; MAPK: mitogen activated protein kinase; ERK: extracellular signal-regulated kinases; Akt: protein kinase B; PI3K: phosphatidylinositol 3-kinase; mTOR: mechanistic target of rapamycin; DAG: diacylglycerol; AA: arachidonic acid; cPLA
2: cytosolic phospholipase A
2; PKC: protein kinase C; PKA: protein kinase A; LPCAT: acetyl-CoA: lyso-PAF acetyltransferases; PAF-CPT: dithiothreitol l-insensitive CDP-choline: 1-alkyl-2-acetyl-
sn-glycerol cholinephosphotransferase; ATP: adenosine triphosphoric acid; cAMP: cyclic adenosine monophosphate; PLC: phospholipase C; MMP: metalloproteinase; COX: cyclooxygenase; iNOS: nitric oxide synthase; eNOS: endothelial nitric oxide synthase; ROS: reactive oxygen species; RNS: reactive nitrogen species; NADPO: nicotinamide-adenine dinucleotide phosphate oxidase; XO: xanthine oxidase; IL-6: interleukin-6; IL-1: interleukin-1; TNFα: tumour necrosis factor-α; ACS: acute coronary syndrome; VEGF: vascular endothelial growth factor; PL: phospholipids; CVD: cardiovascular diseases; CNS: central nervous system.
Signalling through other pathways is also amplified by the PAF/PAF-R pathway activation, since inhibition of PAF synthesis or PAF-R blockade significantly attenuates signalling through apparently unrelated pathways, suggesting a critical role for PAF/PAF-R action as a co-stimulatory signal. For example, many VEGF-directed effects on vascular endothelium require PAF synthesis [
57]. Nevertheless, the activation of the PAF/PAF-R pathway further triggers the activation and aggregation of platelets and leukocytes and promotes leukocyte and platelet adherence, motility, chemotaxis, invasion, migration, ROS generation, and further PAF formation () [
89,
98].
3.1.3. PAF Levels Result from Enzymatic Biosynthesis, Non-Enzymatic Oxidative Synthesis, and Enzymatic Catabolism
Under normal circumstances, homeostatic levels of PAF present in plasma and biological tissue seem to be regulated by a balance of its biosynthetic and catabolic enzymatic pathways [
57]. PAF is synthesised throughout the body by the specific stimulation of various cell types such as platelets, macrophages, monocytes, eosinophils, basophils, and endothelial cells. PAF is mostly produced in the blood, lungs, kidney, myocardium, brain, liver, skin, saliva, retina, uterus, and embryo [
56,
99,
100]. Two enzymatic pathways by which PAF is biosynthesised in the body are the ‘remodelling’ and the ‘
de novo’ pathways ((A1)).
Figure 3. PAF levels result from enzymatic biosynthesis, non-enzymatic oxidative synthesis, and enzymatic catabolism, while bioactive microconstituents of the Med-diet beneficially affect these pathways. (A1) The enzymatic biosynthesis of PAF contributes to basal PAF levels or a periodic increase of PAF levels during normal inflammatory responses, while during unresolved and chronic inflammatory manifestations, the enzymatic biosynthesis of PAF is responsible for pathologically increased PAF levels through a continuous induction of the PAF cycle; (A2) Non-enzymatic synthesis of PAF occurs during oxidative stress, increasing ROS and RNS and inducing the synthesis of PAF and PAF-like molecules. When Ox-LDL is produced, PAF-like molecules mimic the activities of PAF. These pathways are not regulated enzymatically; (B) Catabolism of PAF is enzymatically regulated by PAF-AH. PAF catabolism is activated during both acute and chronic inflammatory manifestations and inactivates both PAF and PAF-like molecules; (C) Bioactive microconstituents present in foods of the Med-diet (i.e., polar lipids) have demonstrated beneficial outcomes by inducing homeostatic equilibration of PAF levels and activities through the Inhibition of the PAF/PAF-R pathways and modulation of the PAF anabolic and catabolic enzymes. PAF: platelet-activating factor; PAF-R: G-protein coupled PAF-receptor; PAF-CPT: dithiothreitol l-insensitive CDP-choline: 1-alkyl-2-acetyl-sn-glycerol cholinephosphotransferase; Lyso-PAF-ATs (LPCAT1, LPCAT2): acetyl-CoA: lyso-PAF acetyltransferases; cPLA2: cytoplasmic phospholipase A2; PAF-AH: PAF-acetylhydrolase; PC: Phosphatidylcholine; ROS: reactive oxygen species; RNS: reactive nitrogen species; LDL: low-density lipoprotein; Ox-LDL: oxidised-LDL; Med-diet: Mediterranean diet.
The remodelling enzymatic pathway of PAF biosynthesis involves remodelling of a membrane lipid constituent (a long-chain fatty acyl residue in
sn-2 is replaced with an acetyl residue), and it has been proposed that this pathway is periodically involved in the acute pro-inflammatory production of PAF under activation of several cells during inflammation [
101]. More specifically, the action of cytoplasmic phospholipase A
2(PLA
2) yields a precursor of PAF called lyso-PAF (1-O-alkyl-
sn-glyceryl-3-phosphorylcholine), which is then acetylated by at least two isoforms of acetyl-CoA: lyso-PAF acetyltransferases, namely, LPCAT1 and LPCAT2 (lyso-PAF AT), leading to the formation of PAF [
102]. LPCAT2 is highly expressed in inflammatory cells, and, depending upon the inflammatory stimulus used to activate the cells, PAF is produced within seconds, minutes, or hours following stimulation. In addition, PAF itself can act as an inflammatory signal, and the binding of PAF to its receptor on inflammatory cells can promote the very rapid (within 30 s) production of PAF; PAF-induced, protein kinase, Cα-mediated phosphorylation of LPCAT2 enhances enzymatic activity, leading to the vary rapid production of PAF. Thus, a PAF cycle can consistently induce increased PAF levels and subsequent inflammatory cascades ( and )
The
de novo enzymatic pathway of PAF biosynthesis is similar but distinct to the biosynthesis of phosphatidylcholine, since a phosphocholine function is transferred to alkyl acetyl glycerol. This pathway has been initially reported as the pathway responsible for the constitutive production of PAF basal levels. A key step in this route is the conversion of 1-O-alkyl-2-
sn-acetyl-glycerol to PAF by a specific dithiothreitol l-insensitive CDP-choline: 1-alkyl-2-acetyl-
sn-glycerol cholinephosphotransferase (PAF-CPT) [
57,
81]. Interestingly, apart from the remodelling pathway, which is always activated in both acute and chronic inflammation, the key enzyme of the ‘
de novo pathway, PAF-CPT, seems to be more active during chronic inflammatory manifestations, thus contributing to an increase of basal levels of PAF that seem to be related to the continuous activation of inflammatory cascades in the long-term during the development of inflammation-related chronic disorders [
57,
70,
81]. Thus, the regulation of the biosynthetic pathways of PAF seems to be more complicated than was initially thought, while both PAF biosynthetic routes are correlated with well-established inflammatory and immunological biomarkers (i.e., several cytokines, viral load, CD-40L, etc.) in several cases [
57,
69,
70,
79,
81,
103,
104].
Apart from its enzymatic biosynthetic pathways, PAF and PAF-like lipids can also be produced through non-enzymatic synthesis by oxidation of other lipids during oxidative stress [
105,
106]. The production of PAF and such PAF-like oxidised lipids usually occurs during inflammation and oxidative stress ((A2)). Vice versa, PAF and PAF-like lipids can also stimulate the production of ROS and nitrogenous species such as reactive nitrogen species (RNS) during oxidative and nitrosative stress in inflammation-induced endothelial dysfunction and atherosclerosis [
89].
The main catabolic enzyme that reduces PAF levels is PAF acetylhydrolase (PAF-AH), delicate phospholipase A
2 that removes the acetate group from the PAF molecule and thus transforms PAF to its inactive form of lyso-PAF (B) [
107]. These enzymes, PAF-AH, are produced largely by hepatocytes and macrophages, and are widely distributed in human plasma, blood cells, and a variety of tissues. Subsequent research revealed that the PAF-AH family includes intracellular forms called PAF-AH I and PAF-AH II, as well as an extracellular third isoform [
108]. PAF-AH, an extracellular isoform in plasma, is a member of the PLA
2 superfamily of enzymes that is also known as lipoprotein-associated phospholipase A
2 (Lp-PLA
2), since it circulates in blood in association with plasma lipoprotein particles such as LDL and HDL, or the PLA
2 group 7 (PLA
2G7) [
107,
108,
109,
110]. Intracellular PAF-AH type I exists in the cytoplasm of many (probably all) types of mammalian cells and tissues [
111]. Interestingly, the intracellular PAF-AH Type II that has no homology with PAF-AH I, but shares sequence similarity to plasma PAF-AH, was reported to act as a cellular Phospholipase A
2 that hydrolyses oxidatively modulated or truncated phospholipids (with short length or oxidatively modified
sn-2 acyl chains). It is thus suggested that PAF-AH (II) functions as an antioxidant phospholipase that plays a protective role also against oxidative stress [
108,
112].
3.2. The PAF Pathway and Metabolism in Chronic Diseases
Under normal conditions, plasma and tissue levels of PAF are tightly regulated by its metabolic pathways. However, production of PAF and PAF-like molecules can become elevated and/or dysregulated during extended periods of immune activation and chronic inflammation-related disorders by amplification of its synthesis, either through cascades activating its biosynthetic enzymes or through oxidative production of PAF, or usually by both [
57,
69,
70,
79,
81,
103,
104,
113]. PAF plays a major role in the physiopathology of inflammatory reactions and is produced and released in large quantities by inflammatory cells in response to specific stimuli, such as upstream regulators (IL-1, IL-6, TNF-α, Endothelin, oxidative stress, and PAF itself; A) [
57,
78,
89,
114].
Increased PAF levels at the site of inflammation can activate several cell types through its receptor. This leads to the initiation of a broad spectrum of PAF effects depending on the cell type and tissue, which is achieved through the production and release of various downstream mediators, such as PAF itself and several other mediators of inflammation such as eicosanoids, cytokines (i.e., TNF-α, IL-1α, IL-6, IL-8, INF-γ, etc.), growth factors (i.e., VEGF, IGF, TGF), ROS, and RNS, but also through the expression of selectins and integrins (i.e., ICAM, VCAM, P-Selectin, E-Selectin) in the membranes of activated cells (B) [
57,
58,
78,
89,
113,
114].
The interconnected crosstalk between PAF, pro-inflammatory upstream mediators that induce PAF production, and PAF-induced downstream mediators seems to be interrelated during inflammatory manifestations and inflammation-related chronic diseases. These pathways serve as one of the main junctions between many inflammatory cascades that ultimately lead to endothelium dysfunction and inflammation-related disorders such as atherosclerosis, CVD, renal disorders, cerebrovascular, central nervous system (CNS) disorders, metastatic angiogenesis during cancer, sepsis, and several other chronic disorders (B) [
57,
58,
78,
89,
113].
3.2.1. PAF in Atherosclerosis and CVD
Cardiovascular diseases (CVD) are the leading cause of death worldwide. It is estimated that 49 million people are now living with the disease in the European Union alone [
115]. Atherosclerosis is a slow progressive disease in which lesions or plaques form in large and medium-sized arteries, consisting of necrotic cores, calcified regions, accumulated modified lipids, migrated smooth muscle cells (SMC), foam cells, endothelial cells, and several leukocyte subtypes. Monocytes, circulating blood precursors of tissue macrophages, and myeloid-derived DC influence plaque development following recruitment into the intima and differentiation to foam cells.
In contrast to the previous notions concerning the passive accumulation of lipids in macrophages during the formation of foam cells, it is now clear that there are more complex inflammatory mechanisms acting on monocytes, macrophages, platelets, several other leucocyte subtypes, and endothelial cells that seem to promote atherosclerosis via pro-inflammatory foam cell formation [
66]. Persistent and unresolved inflammation at the vascular wall gives rise to inappropriate platelet and leukocyte recruitment at the endothelium. The inflammatory interplay and crosstalk between these cells and endothelial cells, facilitated by several inflammatory mediators, initiates the cascades that induce chronic inflammatory manifestations at the vascular wall, which counteracts the homeostatic inflammatory response, leading to endothelial dysfunction and initiation of proatherogenic events that lead to atherogenesis and atherosclerosis [
116]. PAF is one of the main junctions between several inflammatory pathways (cytokines, oxidative stress, eicosanoids, etc.) and their interplay with cells participating in inflammation-related atherosclerosis. Therefore, PAF is implicated in all stages of atherosclerosis, from the initiation of atherogenesis all the way through to plaque formation, development, instability, and rupture [
58,
89,
105,
117].
The Pro-Inflammatory Crosstalk between PAF with Several Cells and the Endothelium Induces Early Pro-Atherogenic Phases of Endothelial Dysfunction
At early pro-atherogenic conditions, PAF is produced in several cells, such as platelets, leukocytes, and endothelial cells under pro-inflammatory stimuli and/or by the oxidation of lipoproteins. Thus, PAF can further propagate oxidative stress, through the oxidation of LDL and the reduction of NO bioavailability, but mostly by acting as a potent chemotactic factor for other human cells that exhibit its receptor on their membranes, such as monocytic and granulocytic leukocytes of the innate and adaptive immune system, endothelial cells, etc. Following these activations, a number of mediators are released by these activated cells (e.g., PAF itself, several cytokines, eicosanoids, ROS, RNS, and several enzymes), while adhesive molecules are expressed in their cell membranes (i.e., chemokines, selectins, and integrins, such as E-selectin, P-selectin, MCP1, ICAM-1, VCAM-1, etc.) that facilitate platelet-platelet, platelet-leukocyte, and platelet-leukocyte-endothelium aggregates and interplay [
58,
89]. The PAF pathway downstream products can further contribute to the propagation of atherosclerosis.
Molecules of the selectin family mediate interactions between platelets and leukocytes, with the endothelium allowing leukocytes and platelets to roll along the vascular endothelium wall. Platelet binding of the endothelium seems to precede the appearance of leukocytes in plaques and induces bidirectional expression of adhesion molecules and the production of monocyte attracting chemokines, such as PAF that plays a central role in cytokine-induced monocyte adherence to endothelium [
58,
89,
117,
118]. Activated platelets that adhere to the inflamed endothelium may enhance leukocyte recruitment, activation, and transmigration, thereby enhancing the inflammatory processes underlying atherosclerosis [
119]. PAF and Leukotriene B4 (LTB4), derived by activated platelets, leukocytes or endothelium, but also thrombin (through PAF and LTB4 pathways), can propagate the activation of platelets and the subsequent activation and adhesion of leucocytes through the interplay of chemokines and their receptors [
117]. An important aspect of this platelet-leucocyte interplay is the diversity of leukocytes recruited by vessel wall adherent platelets, such as the platelet-mediated recruitment of neutrophils, monocytes, DC, T-lymphocytes, B-lymphocytes, and NK-cells to endothelium [
117].
In addition, platelets regulate neutrophil activation through the generation of PAF as a chemoattractant pro-inflammatory lipid [
120]. Activated endothelial cells and platelets generate considerable amounts of PAF, which act cooperatively with other extracellular stimuli to induce full integrin activation and leukocyte arrest [
58,
89,
120]. However, whether PAF mostly originates from activated platelets, endothelial cells or leukocytes are not well defined yet [
120]. Independently of its origin, the presence of PAF activates through its PAF/PAF-R pathways expression of integrin molecules at cell membranes to promote firm adhesion between leukocytes, platelets, and vascular endothelium [
117].
PAF, other vasoactive compounds, angiogenic compounds, and pro-inflammatory mediators, such as arachidonic acid metabolites, histamine, cytokines, chemokines, and proteolytic enzymes, can also be released by mast cells that accumulate in the human arterial intima and adventitia during atherosclerotic plaque progression, and thus aggravate atherogenesis [
8]. Cytokines produced by mast cells may be activated by pro-inflammatory stimuli, including cytokines, hypercholesterolemia, and hyperglycaemia, and trigger the endothelial expression of adhesion molecules such as P-selectin, VCAM-1, and chemokines such as PAF that mediate the recruitment and adhesion of leukocytes [
8].
Similar to other chemoattractants, PAF has been detected in circulation; however, this molecule is mostly cell membrane-associated and operates in a paracrine manner on the G-protein coupled receptors of neighbouring cells [
58,
89,
120]. Thus, PAF is also a main player in juxtacrine signalling and adhesion of leukocytes to other cells, and has also been shown to regulate firm neutrophil adhesion on the surface of immobilised spread platelets [
119,
121]. The level of platelet stimulation impacts directly on neutrophil adhesion to platelets monolayer, upon which neutrophil activity is spatially regulated by PAF generation [
58,
89,
120]. Platelets and activated neutrophils act jointly to induce expression of adhesion molecules, permeability changes, and limit the bioavailability of nitric oxide, altogether aggravating endothelial dysfunction and facilitating subsequent monocyte plaque recruitment [
122].
The Inflammatory Crosstalk Between PAF and Several Cells at the Intima and Subintima Leads to the Induction of Plaque Development and Increased Plaque Growth and Expansion
In the aortic lumen, endothelial cells have been activated by the aforementioned PAF-implicated downstream manifestations, leading to increased endothelium permeability and endothelial dysfunction. Subsequent abnormal recruitment, migration, and infiltration of monocytes then take place in the intima and subintima. Within the intima, monocytes secrete lipoprotein-binding proteoglycans, resulting in increased accumulation of modified LDL, which sustains inflammation. In addition, once in the intima, differentiation factors such as the macrophage colony-stimulating factor (M-CSF) differentiate pro-inflammatory monocytes into inflammatory type macrophages that ingest modified lipoprotein to become foam cells [
59,
123].
Emerging evidence suggests that the role of monocytes and macrophages in atherosclerosis is not simply that of a passive acceptor of lipids [
66]. Apart from their phagocytic roles, macrophages can also instruct or be instructed by other immune cells by producing various immune effector molecules and by acting as antigen-presenting cells (APC). Plaque-related macrophages can have many phenotypes and functions depending on the stage of the disease; several monocyte subtypes exist, and subsequently several pro-inflammatory and anti-inflammatory macrophage subtypes also exist, while macrophages can rapidly adapt their phenotype and consequently their function in response to changes of the microenvironment and intracellular signalling pathways [
122]. After appropriate activation, macrophages can exhibit a pro-inflammatory phenotype that can further activate endothelial cells, which in turn triggers further blood monocyte recruitment [
122,
124]. Thus, upon activation, the pro-inflammatory subtype of macrophages and foam cells produce inflammatory cytokines and chemokines that enhance inflammation and further regulate monocyte and T cell infiltration [
59,
124].
Macrophages express a myriad of receptors including G-protein coupled receptors such as PAF-R, through which they scan their environment for activation or polarisation signals, e.g., cytokines, growth factors, oxidised phospholipids, etc., [
59,
124,
125,
126], while, when in the atherosclerotic plaque, macrophages are capable of releasing a large repertoire of pro-inflammatory cytokines according to their phenotype and depending on the plaque microenvironment, including IL-1, IL-6, IL-12, IL-15, IL-18, TNF family members, and PAF, as well as anti-inflammatory cytokines like IL-10 and TGF-β family members (TGF-β1, BMPs, GDFs) [
58,
59,
124].
Several autacoid molecules of the microenvironment, such as PAF and its receptor, play a significant role in the pro-inflammatory activation of macrophages by oxidative stress and in the uptake of Ox-LDL by macrophages [
125], since Ox-LDL contains inflammatory PAF-like oxidised phospholipids that mimic PAF and interact with these cells [
105]. In addition, autacoids such as PAF and PAF-like molecules in Ox-LDL also play a significant role in the cytoskeletal reorganisation of these cells during differentiations [
127], as macrophages engulf and retain large molecules such as Ox-LDL, oxidised phospholipids, and blood cells, which have also migrated into the intima and sub-intima. The macrophages become lipid-loaded foam cells through phagocytosis, scavenger-receptor mediated uptake, and pinocytosis; the macrophages become lipid-loaded foam cells [
58]. The term ‘foam cells’ both reflects the microscopic appearance of these lipid-laden macrophages and denotes early fatty streak lesions [
122]. This process is outlined in .
Figure 4. A schematic of the key role of PAF in the onset, progression, and expansion of atherosclerotic plaques and their subsequent cardiovascular disorders. Atherosclerotic events take place in four discrete stages (IIa–IV) as follows: (I) Under normal conditions, blood cells roll within the blood stream during physiological blood circulation. Leukocytes scavenge the endothelium by weak adhesion on it and after rolling, return to the blood stream. (IIa) Upstream pro-inflammatory stimuli (cytokines, PAF, etc.) induce PAF synthesis and expression of the PAF-R on the membranes of endothelial and blood cells. (IIb) Binding of PAF to its receptor on the membranes of these cells further induces the PAF cycle-related amplification of the initial inflammatory stimuli, which is achieved through the expression of inflammation-related genes and the subsequent production and release of various downstream mediators, such as PAF itself and several other mediators of inflammation including eicosanoids, cytokines, growth factors, further oxidative stress (ROS, RNS, Ox-LDL, and Ox-PL), and selectins and integrins in the membranes of activated endothelial cells and leukocytes. (III) If unresolved, the PAF cycle-related inflammatory activation of endothelial cells leads to tight adhesion of leukocytes on the activated endothelium and subsequent migration of these leukocytes and Ox-LDL to the subendothelium. There, the crosstalk of key-junction inflammatory mediators such as PAF within the developing plaque microenvironment, with a panel of inflammatory cells of both the innate and adaptive immune system, favours inflammatory phenotypes in these cells and perpetuates a continuous inflammatory milieu, leading to the differentiation of monocytes to macrophages, which engulf Ox-LDL and further transform to foam cells; thus, facilitating the onset, increase, and expansion of atherosclerotic plaque. (IV) Although plaques can grow to a sufficiently large size to compromise blood flow, most of their clinical complications are attributable to arterial occlusion due to plaque erosion or rupture. Vulnerable plaques are typically large, with a necrotic core covered by a thin fibrous cap, and they contain high levels of inflammatory immune cells. Gradually accumulating foam cells die in the intima due to inflammation-induced apoptosis, and when not promptly disposed of, become necrotic, progressively leading to the formation of a thrombogenic and pro-inflammatory necrotic core with cholesterol crystals. In addition, the thin layer of the fibrous cap easily ruptures due to PAF-related inflammatory and atherothrombotic stimuli. Thus, as the plaque continues to develop, it can become unstable and rupture, leading to major cardiovascular event. PAF: platelet-activating factor; PAF-R: G-protein coupled PAF-receptor; ROS: reactive oxygen species; RNS: reactive nitrogen species; Ox-LDL: oxidised LDL; Ox-PL: oxidised phospholipids; IL-6: interleukin-6; IL-1: interleukin-1; TNFα: tumor necrosis factor-α; VEGF: vascular endothelial growth factor.
The interplay of PAF with other APC such as DC is also implicated in several stages of atherosclerosis. Under atherosclerotic conditions, the role of DC is to take up atherosclerosis-specific antigens, which become locally activated, and migrate out of the plaque towards either local draining or distant lymph nodes, where they induce protective anti-inflammatory T cell activation and proliferation. However, apart from their role in directing different T and B cell subsets, not all their functions have been fully elucidated or understood. Nevertheless, impaired migration of DC to lymph nodes results from inhibitory signals generated by PAF or Ox-LDL that act as a PAF mimetic, thus suppressing immunologic priming. In contrast, normal DC migration and priming can be restored by HDL or HDL-associated PAF acetylhydrolase (PAF-AH), which mediates inactivation of PAF and oxidised LDL. In this context, HDL and PAF-AH maintain a normally functional DC compartment [
128]. In addition, DC produce PAF that engage the PAF-R in DC membranes during maturation, and thus the capacity of DC to present antigens to lymphocytes is downregulated, due to the induction of IL-10 and the sustained and increased PGE
2 synthesis mediated by the PAF-R. In contrast, PAF-R antagonists, by disrupting this suppressor pathway, increase DC function and could therefore be useful in increasing efficiency of vaccines and/or treatment [
129]. The above PAF effects on DC perpetuate local inflammation, decrease the activation of anti-inflammatory T-lymphocytes, and thus further increase plaque growth.
Lymphocytes, particularly T-lymphocytes, are also recruited to the vessel wall by mechanisms such as monocyte recruitment; thus, they are present in atherosclerotic lesions in parallel with macrophages, but in lower amounts. CD4+ T cells (also called Th1 cells) express pro-atherogenic roles, whereas prominent Th2 (CD8+ T cells) and Treg responses seem to exhibit unclear and still controversial anti-inflammatory effects, resulting in a reduction of atherosclerosis and/or a more favourable plaque morphology in atherogenesis. PAF and other platelet-related inflammatory mediators, such as thromboxane A
2, serotonin, and histamine, also display Th1 cell-regulatory effects towards the Th1 response that promotes the progression of atherosclerosis and diverse effects on Th2 response [
130]. Activated platelets produce a significant amount of TxA
2, which inhibits Th1 proliferation and cytokine production [
131], while they also express PAF-R, and PAF can enhance Th1 cytokine production [
130,
132].
PAF can also promote differentiation of Th17 cells that are present in atherosclerotic lesions, which can induce cytokine production by these cells. Activated platelets and platelet thrombi create a unique microenvironment with counteracting mediators for Th17 polarisation by secreting substantial amount of PAF, TGFβ, and IL-1β [
130]. However, the role of Th17 also remains controversial, as both atherogenic, as well as atheroprotective, effects have been reported [
59]. Nevertheless, both PAF and Ox-LDL that mimic PAF and the PAF-R have the capacity to induce atherogenesis due to activation of T-cells and monocytes/macrophages [
133]. These events lead to an expansion of atherosclerotic plaque burden and perpetuation of the pathogenic T-cell response.
Overall, there is intricate interplay and crosstalk between a panel of inflammatory cells of both the innate and adaptive immune system. When key-junction inflammatory mediators within the developing plaque microenvironment are increased, there is favour towards inflammatory phenotypes in these cells, which perpetuates a continuous inflammatory milieu, leading to further increase and expansion of the atherosclerotic plaque. Subsequently, the intimal thickness increases, and blood flow is eventually impaired. Gradually accumulating foam cells die in the intima through inflammation induced apoptosis. When these cells are not promptly disposed of they become necrotic, progressively leading to the formation of a thrombogenic and pro-inflammatory necrotic core containing cholesterol crystals [
58].
The Overgrowth and Instability of Plaques and Subsequent Acute Cardiovascular Events
During plaque growth and expansion, SMC migrate from the media to the intima and proliferate, forming a fibrous cap from extracellular matrix deposition, where activated lymphocytes and calcium deposits are found. Although plaques can grow to a sufficiently large size to compromise blood flow, most of their clinical complications are attributable to arterial occlusion due to plaque erosion or rupture. Vulnerable plaques are typically large with a necrotic core covered by a thin fibrous cap and contain high levels of inflammatory immune cells [
122]. The thin fibrous cap easily ruptures, as there are areas of the plaque where few SMC are present, and macrophages exist in abundance. This is because inflammatory cells cause the death of SMC, which are the main source of collagen that produce and maintain the fibrous cap. PAF is also implicated in the release of several proteases from leukocytes, such as elastase, that degrade the vessel’s extracellular matrix components of the intima, which may lead to plaque rupture [
58]. As the plaque continues to develop it can become unstable and rupture, leading to a major cardiovascular event such as myocardial infarction, stroke, or congestive heart failure, depending on the location of the rupture.
Platelets are critical effectors in the development, progression, and resolution of the final stages of atherosclerosis, and plaque rupture, which is responsible for acute coronary disorders and stroke, not only due to their direct effects on the endothelium but also by acting as a ‘bridge’ for other cells within the vascular system [
119,
121]. Plaque rupture occurs under inflammatory cascades and atherothrombosis through an interplay of platelet-leukocyte aggregates. Upon vessel injury (i.e., plaque rupture), platelets readily adhere to damaged endothelium, and this binding event facilitates further activation and discharge of activating factors stored in platelet granules. Such platelet secretory components include membrane ligands and several chemokines such as PAF that play a role in further recruitment of leukocytes, additional platelets, or other blood cells to the vessel wall [
121]. Platelet adhesion under conditions of high shear stress, which occurs in stenotic atherosclerotic arteries, is central to the development of arterial thrombosis. Therefore, precise control of platelet adhesion must occur to maintain blood fluidity and to prevent thrombotic complications [
119].
Concluding Remarks on PAF in Atherosclerosis and CVD
The potent pro-inflammatory mediator, PAF, and its related PAF/PAF-R pathways are key-junctions of the inflammatory milieu during all stages of atherosclerosis and subsequent CVD. Some biochemical mechanisms involved include the pro-inflammatory induction of endothelial dysfunction, oxidative and nitrosative stress, increased platelet reactivity, recruitment/tight-adhesion, and trans-endothelial cell migration of inflammatory cells from the circulation, differentiation of pro-inflammatory monocytes to inflammatory macrophages, induction of macrophage uptake of Ox-LDL, foam cell formation, induction of plaque growth, plaque instability that leads to eventual plaque rupture, and subsequent cardiovascular events. Outcomes from multiple animal model experiments and several clinical studies have also outlined the crucial role of PAF in atherosclerosis due to its elevated levels and its inflammatory interplay and crosstalk with several cells in the pathogenesis of cardiovascular disorders. Clinical studies that have evaluated the role of PAF as a predictor of CVD have also been reviewed [
89].
PAF-R antagonists have been tested with promising results [
134,
135,
136,
137,
138,
139,
140], however the most prominent beneficial outcomes against atherosclerosis development and CVD were found when food-derived PAF inhibitors such as those present in the foods of the Med-diet. These molecules beneficially inhibit PAF activities and modulate its metabolism towards homeostatic PAF levels [
103,
140,
141,
142,
143,
144,
145]. Many of these components are present in olive oil, wine, fish, and dairy products (). Interestingly, the administration of polar lipid extracts from fish or olive oil to hypercholesterolemic rabbits lead to the regression of atherosclerotic plaques [
103,
142,
143,
144,
145]. These results clearly outline that targeting inflammation and its key-junctions such as the PAF/PAF-R pathways and PAF metabolism provide beneficial outcomes against atherosclerosis and CVD, even without targeting hypercholesterolaemia. Thus, by targeting inflammation, the cause of these disorders through non-toxic approaches such as the Med-diet and by not targeting single risk factors (such as hypercholesterolaemia) seems to provide preventive and protective beneficial results against atherosclerosis and CVD.
Table 1. Studies on the beneficial impact of microconstituents from foods of the Mediterranean diet, such as polar lipids and vitamins, towards inflammation-related disorders, through their effects on the PAF pathways and metabolism.
3.2.2. The Role of PAF in Cancer and Metastatic Angiogenesis
Cancer is the second leading cause of death in developed countries. New blood vessel formation penetrating solid tumours seems to be required for their growth and metastasis. Production of PAF and overexpression of PAF-R are implicated in the tumour-endothelium interplay during cancer growth, invasion, and metastasis in several types of cancer [
57,
114,
169,
170,
171]. PAF and PAF-R are also involved in tumour growth that is associated with immunosuppression [
172,
173,
174], while the crosstalk between PAF/PAF-R pathways and growth factors receptors pathways suggests a potentially important signalling link between inflammatory and growth factor signalling in cancer [
173,
174,
175].
It is not yet fully understood whether the initial levels of PAF in the tumour microenvironment originate from migrated inflammatory circulating cells as a response, or by activated endothelial cells in the vessels neighbouring tumours, or by the tumour cells themselves. However, there is correlation between the malignancy of cancer cells and PAF production and PAF-R expression. It seems that the production and accumulation of PAF in the tumour microenvironment originates from the coexistence of two or of all these procedures and/or by the inactivation of PAF-AH. For example, the PAF basic biosynthetic enzymes such as LPCAT1 (an isoform of lyso-PAF-AT) are overexpressed in several cancer cells and correlated with cellular invasiveness and migration. Therefore, LPCAT1 seems to contribute to tumour growth and metastasis in these types of cancer [
176,
177]. Moreover, endothelial cell PAF production results in enhanced inflammatory cell recruitment, while endothelial accumulation of PAF by PAF production and inactivation of PAF-AH plays also a role in cancer cell migration to distal locations [
178]. In addition cigarette smoking, a classic risk factor for several cancers, contributes to metastatic disease via production of PAF and PAF-like molecules in lung tumours [
174], while smoking related inhibition of breast cancer cell PAF-AH results in PAF accumulation and a subsequent increase in cell motility, tumour growth, and metastasis [
178,
179].
Independently of the origin, the presence of PAF in the microenvironment of tumours activates cancer cells and endothelial cells to further amplify the production of both PAF, angiogenic factors, and increased expression of their receptors on cell-membranes, including the PAF-receptor, leading to a PAF cycle and further induction of several PAF/PAF-R related cascades. These cascades, in coordination with angiogenic cytokines and growth factors, enhance the initial signal and induce morphological alterations and cellular activities such as growth proliferation and motility, expression of adhesion molecules, extracellular matrix breakdown, migration, and endothelium reorder that leads to the formation of distinct neoplastic vessels in the tumour microenvironment [
57]. All of the above result in the onset and development of tumour-induced angiogenesis and metastasis [
57]. For example, in pancreatic cancer, PAF overexpression leads to cell proliferation and tumourigenesis through the PAF/PAF-R related MAPK signalling pathway, causing neoplasia [
170]. In addition, PAF and PAF-like molecules are in part transported by tumour-derived extracellular vesicles, which play an important role in intercellular communication through PAF-R expressed by a variety of microenvironmental cells and endothelial cells, favouring metastasis [
172]. Apart from its crucial role in cancer metastasis, which has been extensively reviewed [
57], recent outcomes have demonstrated that PAF is also implicated in immunosuppression-related cancer induced by UV-irradiation, in which UV-induced production of PAF and PAF-like molecules and the expression of PAF-R activates systemic immune suppression and delays DNA repair [
93].
On the other hand, PAF and its receptor have been beneficially associated with cell survival during radiotherapy or chemotherapy, by proliferative signals on the surviving cells that are induced by apoptotic cells. These signals take place through mechanisms dependent on the activation of PAF-R related pathways of NF-kB, such as up-regulation of anti-apoptotic factors and decrease of the cytotoxic effect of chemotherapeutic agents, thereby contributing to cell survival [
172]. However, recent studies have demonstrated that during cancer therapies (i.e., irradiation of carcinoma cells or chemotherapy), PAF-R ligands can be generated that further aggravate immune suppression and, when bound on the PAF-R of cancer cells, induce anti-apoptotic factors that protect the tumor cells from death induced by these treatments, while the combination of radiotherapy with PAF-R antagonists could be a promising strategy for cancer treatment [
173,
180].
In several cancer types, PAF through the NF-kB pathway controls the expression of genes that take part in processes that lead to metastatic angiogenesis on one hand, while on the other hand it results in apoptosis of cancer cells, during the immune response and haematopoiesis during chemotherapies and radiotherapies [
57]. It seems that PAF is a unique growth regulator with apparently diverse functions; PAF, like NF-kB, seems to promote distinct biological processes, and these dual actions of PAF may relate to the point of action in the cell cycle [
57]. The timing, space, and quantity of its production play a significant role in the malignant or beneficial direction of its effects. Understanding how conditions and factors that control timing, location of activity, and the quantity of PAF levels and how these relate to the metabolic enzymes of PAF is of great importance.
PAF-R antagonists have exhibited promising results in vitro and in vivo as anti-angiogenic molecules in several cancer cells and tumours, but also by reducing persistent pain during cancers [
57,
114,
181,
182]. In addition, the combination of chemotherapy and classic PAF-R antagonists seems to reduce the tumour volume and cause higher tumour regression when compared to each treatment alone [
172,
180]. Recently, synthetic glycosylated alkyl-phospholipids that act as PAF agonists and antagonists have exhibited promising antiproliferative outcomes and are now regarded as and can be new class of anti-tumour drugs [
183]. However, apart from using synthetic or classic PAF antagonists, a dietary profile rich in bioactive molecules and food-derived PAF inhibitors such as those present in foods of the Mediterranean diet seems to provide beneficial preventive and protective effects against development, growth, and metastatic manifestations of cancer cells by inhibiting PAF activities and/or modulating its metabolism towards homeostatic PAF levels [
57,
137] ().
3.2.3. The Role of PAF in Glomerulosclerosis and Renal Disorders
PAF has been characterised as one of the main inflammatory mediators implicated in renal pathophysiology [
184]. Production of PAF in the kidney can potentially be attributed to infiltrating inflammatory cells, but mostly to resident renal cells such as the mesangial cells of glomeruli [
81,
185]. Once synthesised, PAF does not accumulate in renal cells, but it is secreted and thus affects mesangial cells, neighbouring podocytes, and other infiltrating cells by binding to its receptor and inducing PAF/PAF-R pathways. In the kidney, PAF-R mRNA is ubiquitously expressed, and a gradient of its expression levels seems to exist; it is higher in the renal cortex, lesser in the outer medulla, and much lesser in the inner medulla, while within the nephron, the glomerulus demonstrates the highest PAF-R expression [
78]. PAF infusion affects renal hemodynamics and glomerular permeability, resulting in changes in filtration rate and proteinuria [
78].
Apart from the physiological effects of PAF, its increased levels and overexpression of PAF-R in kidney are involved in the pathogenesis and progression of renal damage and acute renal failure [
78,
184,
186,
187]. Thus, PAF is implicated in antibody- and complement-mediated glomerular injury, in antithymocyte antibody-induced glomerular damage and other experimental models of immune renal damage, and in patients with lupus nephritis and IgA nephropathy [
78]. PAF participates in the development of kidney graft dysfunction, namely, transplant rejection chronic transplant nephropathy and immunosuppressive drug-mediated nephrotoxicity [
78]. PAF is also implicated in drug-related renal damage of different causes, such as cyclosporin A, glycerol, gentamicin, and cisplatin [
78].
However, the most important role of PAF in renal dysfunction is its implication in the onset and progression of glomerulosclerosis, a renal disorder that shares common features with atherosclerosis and can lead to organ failure. Crosstalk between several renal cells of the glomeruli, such as the mesangial cells and podocytes, takes place during this disorder and the PAF/PAF-R pathways form key junctions during all steps. It has been proposed that PAF might be one of the chemokines released by mesangial cells that mediate their communication with podocytes. PAF enhances its own receptor expression [
188], through which it stimulates multiple downstream inflammatory signalling pathways, mostly in mesangial cells, leading to the release of AA metabolites and subsequent prostanoid and thromboxane generation, leukocyte recruitment, mesangial cell contraction, intracellular lipid accumulation, and transforming growth factor (TGF)-β mediated upregulation of extracellular matrix production. All of these molecular events potentially culminate in the development of glomerulosclerosis and fibrosis, which are key feature of progressive renal disease, regardless of the primary cause [
78,
188,
189]. In addition, PAF promotes inflammatory infiltration of the glomerulus, since it functions as a chemoattractant, and it increases adhesion of polymorphonuclear leukocytes and monocytes to mesangial cells through integrins [
78]. PAF increases the expression of the LDL-receptor and scavenger receptors in mesangial cells, and thus causes an increased uptake of lipids and their accumulation in mesangial cells, leading to the formation of foam cells, which is an important stage of glomerulosclerosis and a key factor that participates in the initiation and progression of lipid-mediated renal injury [
78,
188].
Several PAF-R antagonists have been used in several of the aforementioned renal disorders with promising results [
78,
137]. However, apart from using classic PAF antagonists, recent results have highlighted the protective role of a dietary profile rich in bioactive molecules, antioxidants, and food-derived PAF inhibitors such as those present in the Mediterranean diet through beneficially inhibiting PAF activities and/or modulating its metabolism towards homeostatic PAF levels [
80,
81] (). In addition, the use of vitamin D or vitamin-D analogues as treatment in haemodialysis patients has also exhibited similar beneficial effects, since such a treatment strongly inhibits PAF and thrombin activities, affects PAF metabolism towards equilibrating PAF levels, and reduces circulating levels of IL-8, IL-1β, and TNF-α [
79]. As reducing dietary cholesterol levels may be ineffective, such outcomes have further supported the notion of using full-fat products such as dairy products and non-low-fat products, since the full-fat dairy products exhibit higher bioavailability of high-value nutrients such as bioactive polar lipids and vitamin D, which both possess strong anti-inflammatory and protective properties [
3].
3.2.4. The Role of PAF in Cerebrovascular and Central Nervous System Disorders
PAF and the PAF/PAF-R pathways are also present in the CNS, where they exhibit a number of diverse physiological and pathological functions. PAF is synthesised in neuronal cells throughout the CNS, while these cells also express the PAF-R [
190,
191]. When present at normal concentrations, PAF is a modulator of many CNS processes, ranging from long-term potentiation to neuronal differentiation [
113,
191]. Excessive levels of PAF appear to play an important role in neuronal cell injury and in various inflammation-related CNS pathological conditions, such as neuroinflammatory cascades implicated in depression and neurodegeneration, Alzheimer’s disease, stroke, ischemia-reperfusion injury, spinal cord injury, multiple sclerosis, Parkinson’s disease, neuropathic pain, epilepsy, central malaria, meningitis, depression, cognitive deficits, and HIV-induced neurotoxicity [
190,
191,
192]. Increased PAF synthesis through the PAF/PAF-R pathways can cause a severe inflammatory response, reduction of biological membrane integrity, ROS and RNS formation, expression and release of cytokines, alterations in blood–brain barrier permeability and the permeability of blood vessel walls, activation and recruitment of inflammatory and immune cells, secretion of cell-specific proteins, induction of cell apoptosis through specific signalling pathways, and other pathological responses [
113,
190,
191,
192,
193]. PAF accumulation in CNS diseases exacerbates the inflammatory response and pathological consequences, while application of PAF inhibitors or PAF-R antagonists significantly reduces inflammation, protects cells, and improves the recovery of neural functions by blocking the PAF pathway [
191,
192,
194]. Several PAF inhibitors of natural origin have also exhibited beneficial outcomes in CNS disorders, especially ginkgolides that are derived from
Ginkgo biloba [
137,
195]. However, further studies are required to establish the mechanisms surrounding how a healthy diet can improve systemic inflammation associated with the PAF pathway and CNS disorders.
3.2.5. The Role of PAF in Allergies and Asthma
Anaphylaxis is defined as a severe, life-threatening, systemic or general, immediate reaction of hypersensitivity, with repeatable symptoms caused by a dose of stimulus that is well tolerated by healthy persons [
196,
197]. Recently, PAF and PAF-AH have been reported as clinically valuable biomarkers of anaphylaxis [
196], since PAF produced and released by mast cells, basophils, neutrophils, eosinophils, fibroblasts, platelets, endothelial cells, and even cardiac muscle cells plays an important role in anaphylaxis and several other allergic reactions, from allergic rhinitis to asthmatic complications [
67,
196,
197,
198,
199,
200,
201,
202]. Eosinophils, mast cells, and basophils are implicated in allergies, and they have the capacity to influence each other’s functions through a crosstalk, where other mediators such as PAF are also implicated [
198,
199,
200,
203]. PAF increases the production of eicosanoids, ROS, cytokines, growth factors, platelet-derived growth factor (PDGF), RANTES, and degranulation of eosinophils, while it also acts as a chemoattractant for these cells, and, via integrins, it increases their adhesion to vascular endothelium. Mast cells not only produce PAF, but they can also be activated by it through the PAF/PAF-R pathways. Thus, exposure of mast cells to PAF leads to the induction of specific functions in these cells such as degranulation of their granules via neuropeptides and PAF-dependent release of histamine. In fact, the greater the levels of PAF in mast cells microenvironment, the more enhanced the release of histamine. At the same time, PAF-activated myocardial mast cells locally release factors responsible for cardiac dysfunction and hypotension that occur in severe anaphylactic reactions [
197,
200].
Increased levels of PAF correlate with the severity of allergic systemic reactions. Thus, PAF has been found to be involved in several allergic and anaphylactic reactions and shock, in inflammation of bronchi and bronchial asthma and in asthmatic patients’ bronchoconstriction, in mucus hypersecretion, in allergic rhinitis, and in urticaria pathogenesis [
200]. Several studies have shown that PAF can enhance obstructive changes of bronchi by stimulation of allergic inflammation of the respiratory tract epithelium, while PAF can also increase the permeability of skin’s capillaries and induces the development of wheals, flare, and inflammatory reactions in the skin through its interactions and crosstalk of the aforementioned inflammatory cells involved in these pathological conditions [
200].
The protective role of PAF-AH in reducing PAF levels is usually highly diminished through allergic reactions [
196,
200], while administration of recombinant PAF-AH in animal models exhibited protective results and reduced mortality due to anaphylactic reactions [
196], implying that modulation of PAF metabolism towards homeostatic PAF levels can also provide beneficial outcomes in these disorders too. In addition, specific PAF-R inhibitors have been used in several allergy-related disorders [
137], and even specific anti-allergic drugs were designed and are currently used according to their anti-PAF effects [
204,
205], while combination of PAF inhibitors with other therapies such as antihistamines provided better outcomes [
137,
198,
199,
201]. However, further studies are required to establish the potential of a healthy diet to improve systemic inflammation associated with the PAF pathway and allergic complications.
3.2.6. The Role of PAF in Chronic Infections and Inflammation-Associated Comorbidities
Inflammatory and immune responses are central to protecting against most infectious agents. However, the pathogenesis and tissue damage after infection are not usually related to the direct action microorganisms and of their replication, but instead to altered immune and inflammatory responses triggered following contact with the pathogen. Many diseases develop as an adverse consequence of an imbalanced inflammatory response; thus, chronic and unresolved infections are usually accompanied by chronic and unresolved inflammatory manifestations and comorbidities [
206]. PAF and PAF-like molecules are implicated in inflammatory manifestations occurring in several infections [
206,
207], such as HIV [
69,
70,
71,
72,
73,
74,
85], leishmaniosis [
208], periodontitis [
75,
76,
77], or even in sepsis [
67,
209]. The relationship between increased PAF levels, overexpression of PAF-R, and the PAF/PAF-R pathways with several other mediators such as cytokines and inflammatory cells leads to the progression of such diseases and their related comorbidities.
The most common coexistent diseases associated with chronic infections are CVD, CNS disorders, and tumour malignancies, which are usually promoted by increased levels of PAF and PAF-related continuous and unresolved inflammation [
57,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
85,
208]. In addition, PAF seems to act in synergy with infectious agents to initiate and propagate the disease process, i.e., viral load in HIV-infected patients was positively correlated with PAF synthesis and levels, while viral products such as Tat-protein induce PAF synthesis and PAF-related HIV-induced non-AIDS comorbidities, such as CVD, Kaposi sarcoma, neurodegeneration, and dementia [
69,
70,
71,
72,
73,
74].
Several PAF inhibitors have been used in infectious diseases with promising results, mostly in relation to their deterioration of the PAF-related chronic inflammatory manifestations [
67,
71,
74,
85,
137,
207,
209,
210,
211]. However, in the case of severe sepsis, clinical trials using recombinant human PAF-AH or PAF-R antagonists failed to reduce the mortality of severe septic patients, although a substantial reduction in organ dysfunction was achieved [
206]. Drugs administrated in such infectious pathologies have also been thoroughly screened for potential dual actions against both the infectious agent and PAF activities and synthesis. Several antiretrovirals and their combinations in highly active antiretroviral therapy have been found to exhibit beneficial outcomes in HIV infected patients through their capabilities to inhibit PAF activities and to influence PAF metabolism towards reduction of PAF levels in vitro and in vivo, while similar outcomes have also been found for several antibiotics [
68,
69,
71,
73]. Nevertheless, inhibition of PAF activities and modulation of PAF metabolism towards homeostatic PAF levels seem to be useful therapeutic targets with which to interfere with inflammatory damage that follows an infection, and thus they may reduce the risk of several comorbidities in infectious disorders. Although there are several studies published on the importance of a healthy diet for infection prevention, further studies are required to establish the potential role of healthy eating to improve systemic inflammation associated with the PAF pathway and related complications during chronic infections.
3.2.7. The Role of PAF in Various Inflammation-Related Chronic Diseases
PAF has also played a role in several other inflammation-related chronic diseases and their related comorbidities, including types I and type II diabetes mellitus [
212,
213,
214], acute pancreatitis [
215,
216], liver injury [
217], inflammation-related intestine tissue dysfunction such as necrotising enterocolitis [
218,
219], inflammatory ocular diseases [
220], vascular dysfunction during acute lung injury [
221], and autoimmune disorders, such as rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis, inflammatory bowel disease, and Crohn’s disease [
222,
223,
224].
Several PAF inhibitors have been used in these inflammation-related diseases with promising results [
137,
216,
217,
222,
225,
226]. These effects were mostly due to the deterioration of PAF-related chronic inflammatory manifestations present in these disorders. However, apart from using synthetic or classic PAF antagonists, a dietary profile rich in bioactive molecules, antioxidants, and food-derived PAF inhibitors such as those present in foods of the Mediterranean diet may provide beneficial preventive and protective effects against these diseases too, through beneficially inhibiting PAF activities and/or modulating its metabolism towards homeostatic PAF levels. For example, the consumption of components of the Med-diet or a traditional Greek Mediterranean diet can reduce PAF-related inflammatory outcomes such as platelet activity in patients suffering from type II diabetes mellitus and metabolic syndrome, but also in healthy subjects. This has been attributed to the presence of PAF inhibitors among other possible effects, and these effects can occur over a short period of time [
227,
228,
229]. In addition, the use of probiotics has exhibited beneficial effects against necrotising enterocolitis [
218]; this is unsurprising, as fermented dairy products, which are also components of the Med-diet, are rich in PAF inhibitors and have also exhibited beneficial outcomes in several inflammation-related intestine dysfunctions [
3].
3.3. Targeting the PAF Pathways and Metabolism – Beneficial Outcomes of the Mediterranean Diet
Common junctions in the mechanistic crosstalk of inflammatory mediators, signalling pathways, and cellular interactions that occur during chronic and unresolved inflammatory manifestations seem to be promising therapeutic targets for the prevention and treatment of inflammation-related chronic diseases. Drug-based therapeutic interventions targeting inflammatory mediators such as cytokines (i.e., by using specific antibodies against pro-inflammatory cytokines and their receptors) and eicosanoids (i.e., by using specific inhibitors of COX-1 and COX-2) have also been proposed, and relative trials such as CANTOS are still in progress. However, such approaches can sometimes provide undesirable effects and may leave the individual immunocompromised and at a greater risk of infections, since disruption of the physiological balance seems to be a risky strategy [
230,
231], which is clearly behind the multifaceted effects of such mediators.
On the other hand, since PAF and its related inflammatory cascades belong to the most vital joint mechanistic pathways of inflammation-related chronic disorders, the exploration of possible therapeutic approaches targeting PAF and its related pathways may provide better outcomes. Focus initially was given to the PAF/PAF-R interaction, thus inhibiting the exacerbation of the complex PAF inflammatory pathways [
89,
134,
135,
136,
137]. There are several agonists of synthetic and natural origin [
57,
89,
134,
135,
136,
137,
140,
232], which can competitively or noncompetitively displace PAF from its binding sites on PAF-R and thus directly inhibit the PAF/PAF-R related pathways and PAF activities. Furthermore, other similar molecules can indirectly affect the PAF/PAF-R pathways by affecting the up-stream and/or downstream microenvironment of PAF-R, lipid, rafts, and other related cellular receptors.
Even though such specific PAF antagonists for the PAF/PAF-R pathway have exhibited promising results, the most prominent beneficial effects have been derived from polar lipids and polar lipid extracts derived from several foods, particularly from foods in the Med-diet (B and ) [
56,
57,
80,
81,
103,
142,
146,
147,
148,
149,
150,
151,
152,
153,
154,
155,
156,
157,
158,
159,
162,
163,
164,
165,
233,
234]. These Med-diet polar lipids exhibit in vitro and in vivo anti-inflammatory activities through either directly or indirectly inhibiting the PAF/PAF-R pathways and thus PAF activities, but also by downregulating its levels through modulating the activities of key metabolic enzymes of PAF by either upregulation of the PAF catabolic enzymes and/or the downregulation of the basic PAF biosynthetic enzymes (C and C, and ) [
57,
80,
81,
103,
146,
148].
Notably, the uptake of such dietary polar lipids seems to beneficially affect the functionality of HDL lipoproteins, especially in atherosclerotic conditions. HDL has been characterised as the ‘good’ cholesterol, since not only does it remove excess cholesterol from the blood stream and from atherosclerotic plaques, but it has also exhibited anti-inflammatory and antioxidative properties through a plethora of cardioprotective enzymes bonded in HDL, including the aforementioned PAF-AH enzyme activity, which is the main catabolic enzyme of PAF [
110]. These HDL-associated activities contribute to the maintenance of endothelial cell homeostasis, which protects the cardiovascular system [
235]. Plasma PAF-AH is also found in atherosclerotic lesions, since it comigrates there along with the lipoproteins (i.e., LDL), where it is incorporated. Plasma PAF-AH (Lp-PLA
2) mainly plays an anti-inflammatory role in leukocyte/platelet/endothelium activation and seems to suppress atherogenic changes in plasma lipoproteins (such as LDL) by promoting the catabolism of PAF and by removing oxidised phospholipids present in Ox-LDL, including oxidised phospholipids that mimic PAF, which are generated by oxidative modifications of lipoproteins such as LDL during pro-atherogenic and atherosclerotic events [
107,
109,
110]. Thus, during inflammatory cascades that cause increased PAF levels, this isoform of PAF-AH (LpPLA
2) seems to be activated as a homeostatic mechanism to downregulate these events by downregulating the levels of PAF and oxidised phospholipids as a terminator signal [
236]. However, during persistent and prolonged inflammatory cascades and persistent oxidation of plasma lipoproteins, plasma PAF-AH is progressively inactivated (plasma PAF-AH is incorporated mainly in LDL) and loses its capacity to protect against the pro-inflammatory actions of PAF and PAF-like lipids [
98]. Because of that, but also because of the activities of the oxidised sub products of PAF-AH actions in LDL oxidised phospholipids, the use of plasma PAF-AH as an atherogenic biomarker and therapeutic target has been debated [
109,
236].
Nevertheless, HDL and its enzymes, including PAF-AH, seem to protect against these manifestations. The focus has been placed on increasing HDL levels as one of the main goals of dietary interventions and drug administration for cardioprotection [
110]. Dietary intake of bioactive polar lipids, particularly those baring ω-3 PUFAs, increase HDL levels and the incorporation of such anti-inflammatory and antioxidant dietary polar lipids to HDL, thus providing an additional protective mechanism by increasing plasma PAF-AH activity and protecting the HDL enzymes (such as PAF-AH) from oxidation-related inactivation. This is in agreement with the beneficial in vitro and in vivo effects of several dietary polar lipids, especially on PAF metabolism and HDL biofunctionality [
56].
PAF can generate ROS, and oxidative stress is a key feature of the atherothrombotic processes in the pathology of CVD. Therefore, it is important to recognise that foods of the Med-diet such as fruit and vegetables are high in chemical constituents, many of which are regarded as powerful antioxidants, such as vitamins A, C, and E [
237]. Despite positive findings from in vitro studies, clinical trials have consistently failed to show a benefit for the use of antioxidants, as associations between plasma concentrations of antioxidant vitamins and protection against CVD have proved elusive, and large interventional trials have failed to conclusively show any benefit of their administration [
238,
239,
240,
241]. Despite this, the European prospective investigation into cancer and nutrition (EPIC) Norfolk study found that increased plasma concentrations of vitamin C were inversely associated with CVD-related mortality and all-cause mortality. The study found that this increase was due to increased intake of fruit and vegetables, which led to an approximate 20% decrease in CVD mortality [
242]. However, a meta-analysis has shown that vitamin C supplementation did not reduce cardiovascular events; thus, the antioxidant effects of vitamin C were not responsible for the beneficial effects of increased consumption of fruit and vegetables [
243]. A large-scale, 20-year study found that diets rich in vitamin C were associated with a lower incidence of stroke, but no coronary heart disease in the elderly [
244]. Considering these findings, it may be the case that vitamin C may not be the active agent that induced the effects witnessed in the Norfolk study, but although eating fruit and vegetables will increase plasma vitamin C levels, the effects observed may be through other fruit- and vegetable-derived nutrients [
241], or synergism between multiple nutrients that affect different mechanisms including inflammation through the mechanisms of the PAF pathways [
245].
The bioavailability of vitamins, phenolic compounds, and other antioxidants is often cited as the main reason that in vitro and ex vivo studies do not seem to agree [
237]. For instance, some antioxidants such as phenolic compounds are effectively screened out by the gut of rapidly metabolised and excreted [
246]. Plasma concentrations of phenolic compounds are typically in the nanomolar range—too low to have a direct impact on antioxidant capacity [
241]. However, many of these antioxidant molecules do seem to possess beneficial effects upon consumption, including the idea that they induce indirect antioxidant activity by acting as a mild toxin to stimulate a general xenobiotic and/or an antioxidant response [
237]. Further research is required to elucidate the effects of certain biomolecules against ROS and inflammatory pathways.
Overall, the protective outcomes of the adoption of Med-diet towards chronic diseases seem to be associated with the pleiotropic beneficial effects of its bioactive microconstituents that are not only limited to increasing plasma-HDL levels, functionality, and providing better stability against oxidation, but mainly on their effects on the levels, activities, and metabolism of key-inflammatory mediators such as PAF [
56,
57]. However, more in vivo results are needed in several chronic disorders and their inflammation-related manifestations in order to further support these findings. In particular, clinical trials implementing dietary patterns such as the Med-diet that are rich in bioactive polar lipids interacting with the PAF/PAF-R pathways and metabolism are required to gain further insight into the role of PAF in chronic diseases.
4. Conclusions
In this review, we clarify the roles of risk factors, such as plasma cholesterol and the importance of causative agents for chronic diseases, namely chronic and unresolved inflammation and its manifestations. Instead of cholesterol, targeting and treatment of inflammation will lead to lower side effects in chronic disorders. The overall outcomes and the extensive paradigms of the beneficial effects of the Mediterranean diet against the inflammatory milieu, without any reported side effects so far, have radically shifted attention away from the lipid-centric hypotheses and the subsequent trends for targeting cholesterol towards more effective approaches against inflammation, which is the causative factors of chronic diseases.
Therefore, the causative role of inflammation in the onset and progression of several chronic disorders is summarised with respect to the role of PAF and its related inflammatory cascades, which seem to serve as common junctions of the inflammatory milieu. The coexistence of several risk factors seems to upstream pro-inflammatory stimuli (i.e., cytokines, oxidative stress, PAF itself, etc.), leading to increased levels of PAF that, through the PAF/PAF-R pathways, attenuate the initial signal, while downstream inflammatory cascades, in combination with inactivation of homeostatic mechanisms such as PAF catabolism, can result in chronic and unresolved inflammatory manifestations and related chronic disorders.
Common junctions, such as PAF and its related inflammatory pathways, seem to be promising therapeutic targets for the prevention and treatment of the onset and progression of inflammation-related chronic diseases, particularly CVD. Implementation of healthy lifestyle choices based on appropriate dietary interventions and exercise have exhibited beneficial outcomes and health benefits, without noticeable side effects. Adoption of dietary patterns such as the Med-diet provides bioactive food microconstituents with pleiotropic beneficial effects that are not limited to decreasing co-absorption of cholesterol and increasing plasma HDL levels and functionality, but mainly by providing better stability against oxidation and inflammation. Therefore, microconstituents such as polar lipids and vitamins present in foods of the Med-diet beneficially affect the levels, activities, and metabolism of key inflammatory mediators implicated in chronic diseases, including the PAF pathway, towards reducing inflammation and acquiring homeostasis, which can lead to reduced risk of inflammation-related chronic disorders.
Nature has provided us with a wide range of dietary weapons, which, if appropriately combined in dietary patterns such as the Med-diet, can beneficially contribute to improving our quality of life, health, and life expectancy by equilibrating the inflammatory milieu to normal levels and thus preventively reducing the risk of inflammation-related chronic disorders. Let us not forget the words of Hippocrates of Kos (460-377 BC), who is universally recognised as the father of modern medicine: “Let food be thy medicine and medicine be thy food”.
Author Contributions
A.T, R.L., and I.Z. contributed equally to the drafting of the manuscript.
Acknowledgments
The authors acknowledge the financial support of Enterprise Ireland (study grant references: IP-2016-0488Y; IP-2017-0596-Y; and IP-2017-0508-Y), the Department of Biological Sciences, and the Faculty of Science and Engineering at the University of Limerick, Ireland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huff, T.; Jialal, I.I. Physiology, Cholesterol; StatPearls Publishing: Orlando, FL, USA, 2017. [Google Scholar]
- Cox, R.A.; García-Palmieri, M.R. Cholesterol, triglycerides, and associated lipoproteins. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Emerging risk biomarkers in cardiovascular diseases and disorders. J. Lipids 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S. Risk Assessment and Guidelines for the Management of High Blood Cholesterol. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Schaefer, E.J.; Tsunoda, F.; Diffenderfer, M.; Polisecki, E.; Thai, N.; Asztalos, B. The Measurement of Lipids, Lipoproteins, Apolipoproteins, Fatty Acids, and Sterols, and Next Generation Sequencing for the Diagnosis and Treatment of Lipid Disorders. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Iqbal, F.; Baker, W.S.; Khan, M.I.; Thukuntla, S.; McKinney, K.H.; Abate, N.; Tuvdendorj, D. Current and future therapies for addressing the effects of inflammation on HDL cholesterol metabolism. Br. J. Pharmacol. 2017, 174, 3986–4006. [Google Scholar] [CrossRef] [PubMed]
- Spinas, E.; Kritas, S.K.; Saggini, A.; Mobili, A.; Caraffa, A.; Antinolfi, P.; Pantalone, A.; Tei, M.; Speziali, A.; Saggini, R.; et al. Role of mast cells in atherosclerosis: A classical inflammatory disease. Int. J. Immunopathol. Pharmacol. 2014, 27, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Paschou, S.A.; Goulis, D.G.; Athyros, V.G.; Karagiannis, A. Dietary management of dyslipidaemias. Is there any evidence for cardiovascular benefit? Maturitas 2018, 108, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Pitsavos, C.; Panagiotakos, D.B.; Menotti, A.; Chrysohoou, C.; Skoumas, J.; Stefanadis, C.; Dontas, A.; Toutouzas, P. Forty-Year Follow-Up of Coronary Heart Disease Mortality and Its Predictors: The Corfu Cohort of the Seven Countries Study. Prev. Cardiol. 2003, 6, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Lanti, M.; Kromhout, D.; Blackburn, H.; Nissinen, A.; Dontas, A.; Kafatos, A.; Nedeljkovic, S.; Adachi, H. Forty-year coronary mortality trends and changes in major risk factors in the first 10 years of follow-up in the seven countries study. Eur. J. Epidemiol. 2007, 22, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Kromhout, D.; Blackburn, H.; Fidanza, F.; Buzina, R.; Nissinen, A. Food intake patterns and 25-year mortality from coronary heart disease: Cross-cultural correlations in the Seven Countries Study. Eur. J. Epidemiol. 1999, 15, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Keys, A.; Blackburn, H.; Kromhout, D.; Karvonen, M.; Nissinen, A.; Pekkanen, J.; Punsar, S.; Fidanza, F.; Giampaoli, S.; et al. Comparison of multivariate predictive power of major risk factors for coronary heart diseases in different countries: Results from eight nations of the Seven Countries Study, 25-year follow-up. J. Cardiovasc. Risk 1996, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. Cholesterol Lowering Drugs. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ravnskov, U.; Diamond, D.M.; Hama, R.; Hamazaki, T.; Hammarskjold, B.; Hynes, N.; Kendrick, M.; Langsjoen, P.H.; Malhotra, A.; Mascitelli, L.; et al. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: A systematic review. BMJ Open 2016, 6, e010401. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Q.; Cui, L.H.; Li, C.C.; Yu, Z.; Piao, J.M. Effects of Serum Triglycerides on Prostate Cancer and Breast Cancer Risk: A Meta-Analysis of Prospective Studies. Nutr. Cancer 2016, 68, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D. Serum cholesterol in cross-cultural perspective. The Seven Countries Study. Acta Cardiol. 1999, 54, 155–158. [Google Scholar] [PubMed]
- Papandreou, C.; Tuomilehto, H. Coronary heart disease mortality in relation to dietary, lifestyle and biochemical risk factors in the countries of the Seven Countries Study: A secondary dataset analysis. J. Hum. Nutr. Diet. 2014, 27, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tektonidis, T.G.; Åkesson, A.; Gigante, B.; Wolk, A.; Larsson, S.C. A Mediterranean diet and risk of myocardial infarction, heart failure and stroke: A population-based cohort study. Atherosclerosis 2015, 243, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Ruiz-Canela, M.; Hruby, A.; Liang, L.; Trichopoulou, A.; Hu, F.B. Intervention Trials with the Mediterranean Diet in Cardiovascular Prevention: Understanding Potential Mechanisms through Metabolomic Profiling. J. Nutr. 2016, 146, 913S–919S. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, H.E.; Koeller, E.; Greer, N.; MacDonald, R.; Kane, R.; Wilt, T.J. Effects on Health Outcomes of a Mediterranean Diet With No Restriction on Fat Intake: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 165, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Sanches Machado d’Almeida, K.; Ronchi Spillere, S.; Zuchinali, P.; Correa Souza, G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Barak, Y.; Fridman, D. Impact of Mediterranean Diet on Cancer: Focused Literature Review. Cancer Genom. Proteom. 2017, 14, 403–408. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Mediterranean dietary pattern, inflammation and endothelial function: A systematic review and meta-analysis of intervention trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Kwan, H.Y.; Chao, X.; Su, T.; Fu, X.; Tse, A.K.; Fong, W.F.; Yu, Z.L. The anticancer and antiobesity effects of Mediterranean diet. Crit. Rev. Food Sci. Nutr. 2017, 57, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Zappala, G.; Bernardini, S.; Giambini, I.; Bes-Rastrollo, M.; Martinez-Gonzalez, M. Adherence to the Mediterranean diet is inversely associated with metabolic syndrome occurrence: A meta-analysis of observational studies. Int. J. Food Sci. Nutr. 2017, 68, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvado, J.; Guasch-Ferre, M.; Lee, C.H.; Estruch, R.; Clish, C.B.; Ros, E. Protective Effects of the Mediterranean Diet on Type 2 Diabetes and Metabolic Syndrome. J. Nutr. 2016. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Archundia Herrera, M.C.; Subhan, F.B.; Chan, C.B. Dietary Patterns and Cardiovascular Disease Risk in People with Type 2 Diabetes. Curr. Obes. Rep.2017, 6, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Jannasch, F.; Kroger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Dow, C.; Mancini, F.; Rajaobelina, K.; Boutron-Ruault, M.C.; Balkau, B.; Bonnet, F.; Fagherazzi, G. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 2018, 33, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, L.; Williams, E.J.; Scott, H.A.; Berthon, B.S.; Jensen, M.; Wood, L.G. Diet and Asthma: Is It Time to Adapt Our Message? Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The effects of the Mediterranean diet on rheumatoid arthritis prevention and treatment: A systematic review of human prospective studies. Rheumatol. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Suarez, M.; Boque, N.; Del Bas, J.M.; Mayneris-Perxachs, J.; Arola, L.; Caimari, A. Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. 2017, 37, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, R.; Lewis, J.D. The Role of Diet in Inflammatory Bowel Disease. Curr. Gastroenterol. Rep. 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Aridi, Y.S.; Walker, J.L.; Wright, O.R.L. The Association between the Mediterranean Dietary Pattern and Cognitive Health: A Systematic Review. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary Patterns and Risk of Dementia: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef] [PubMed]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Andrade, J.P. Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review. Oxid. Med. Cell. Longev. 2017, 2017, 6469138. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Notara, V.; Kouvari, M.; Pitsavos, C. The Mediterranean and other Dietary Patterns in Secondary Cardiovascular Disease Prevention: A Review. Curr. Vasc. Pharmacol. 2016, 14, 442–451. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA2004, 292, 1433–1439. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wilmot, K.A.; Ghasemzadeh, N.; Molloy, D.L.; Burkman, G.; Mekonnen, G.; Gongora, M.C.; Quyyumi, A.A.; Sperling, L.S. Mediterranean Dietary Patterns and Cardiovascular Health. Annu. Rev. Nutr. 2015, 35, 425–449. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation 2018. [Google Scholar] [CrossRef] [PubMed]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Chiva-Blanch, G.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; Lapetra, J. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: A prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016, 4, 666–676. [Google Scholar] [CrossRef]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals: A randomized controlled trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive oil, the Mediterranean diet, and arterial blood pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The Implication of platelet-activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Demopoulos, C.A.; Karantonis, H.C.; Antonopoulou, S. Platelet activating factor—A molecular link between atherosclerosis theories. Eur. J. Lipid Sci. Technol.2003, 105, 705–716. [Google Scholar] [CrossRef]
- Legein, B.; Temmerman, L.; Biessen, E.A.L.; Lutgens, E. Inflammation and immune system interactions in atherosclerosis. Cellular Mol. Life Sci. 2013, 70, 3847–3869. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; True, H.D.; Patel, J. Leukocyte Trafficking in Cardiovascular Disease: Insights from Experimental Models. Mediat. Inflamm. 2017, 2017, 9746169. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and Inflammatory Mechanisms of Atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Hansson, G.K. Inflammation and Immunity in Diseases of the Arterial Tree: Players and Layers. Circ. Res. 2015, 116, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 2017, 174, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Feng, Y. Hypercholesterolemia Tunes Hematopoietic Stem/Progenitor Cells for Inflammation and Atherosclerosis. Int. J. Mol. Sci. 2016, 17, 1162. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Grunfeld, C. The Effect of Inflammation and Infection on Lipids and Lipoproteins. In Endotext; De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Angelovich, T.A.; Hearps, A.C.; Jaworowski, A. Inflammation-induced foam cell formation in chronic inflammatory disease. Immunol. Cell Biol. 2015, 93, 683. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.C.; Weyrich, A.S.; Zimmerman, G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie 2010, 92, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Lioni, A.; Tsekes, G.; Demopoulos, C.A.; Lazanas, M.C. In vitro anti-inflammatory and anti-coagulant effects of antibiotics towards Platelet Activating Factor and thrombin. J. Inflamm. 2011, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, V.D.; Chini, M.; Mangafas, N.; Stamatakis, G.M.; Tsogas, N.; Tsoupras, A.B.; Psarra, K.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; et al. In vivo effect of two first-line ART regimens on inflammatory mediators in male HIV patients. Lipids Health Dis. 2014, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Chini, M.; Mangafas, N.; Tsogas, N.; Stamatakis, G.; Tsantila, N.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; et al. Platelet-Activating Factor and Its Basic Metabolic Enzymes in Blood of Naive HIV-Infected Patients. Angiology 2012, 63, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Fragopoulou, E.; Nomikos, T.; Lioni, A.; Mangafas, N.; Demopoulos, C.A.; Antonopoulou, S.; Lazanas, M.C. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): A new insight in the drug therapy of HIV infection? AIDS Res. Hum. Retroviruses 2008, 24, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.; Tsoupras, A.B.; Mangafas, N.; Tsogas, N.; Papakonstantinou, V.D.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; Lazanas, M.C. Effects of highly active antiretroviral therapy on platelet activating factor metabolism in naive HIV-infected patients: II) study of the abacavir/lamivudine/efavirenz HAART regimen. Int. J. Immunopathol. Pharmacol. 2012, 25, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Chini, M.; Tsoupras, A.B.; Mangafas, N.; Tsogas, N.; Papakonstantinou, V.D.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; Lazanas, M.C. Effects of HAART on platelet-activating factor metabolism in naive HIV-infected patients I: Study of the tenofovir-DF/emtricitabine/efavirenz HAART regimen. AIDS Res. Hum. Retroviruses 2012, 28, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Mangafas, N.; Demopoulos, C.A.; Lazanas, M.C. In vivo effects of a Ginkgo biloba extract on platelet activating factor metabolism in two asymptomatic HIV-infected patients. Eur. J. Inflamm.2011, 9, 107–116. [Google Scholar] [CrossRef]
- McManus, L.M.; Pinckard, R.N. PAF, a putative mediator of oral inflammation. Crit. Rev. Oral Biol. Med. 2000, 11, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Antonopoulou, S.; Baltas, G.; Samiotaki, M.; Panayotou, G.; Kotsifaki, H.; Mantzavinos, Z.; Demopoulos, C.A. Isolation and identification of hydroxyl–platelet-activating factor from natural sources. Life Sci. 2006, 79, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, S.; Tsoupras, A.; Baltas, G.; Kotsifaki, H.; Mantzavinos, Z.; Demopoulos, C.A. Hydroxyl-platelet-activating factor exists in blood of healthy volunteers and periodontal patients. Mediat. Inflamm. 2003, 12, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, A.; Korstanje, R. The Role of Platelet-Activating Factor in Mesangial Pathophysiology. Am. J. Pathol. 2015, 185, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol effects on activities and metabolism of Platelet Activating Factor and on inflammatory cytokines in hemodialysis patients. Int. J. Artif. Organs2013, 36, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C. In vitro protective effects of olive pomace polar lipids towards platelet activating factor metabolism in human renal cells. Curr. Top. Nutraceutical Res. 2011, 9, 105. [Google Scholar]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Dalbeni, A.; Giollo, A.; Tagetti, A.; Atanasio, S.; Orsolini, G.; Cioffi, G.; Ognibeni, F.; Rossini, M.; Minuz, P.; Fava, C.; et al. Traditional cardiovascular risk factors or inflammation: Which factors accelerate atherosclerosis in arthritis patients? Int. J. Cardiol. 2017, 236, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Grassia, G.; Botha, S.; Sattar, N.; Maffia, P. Targeting inflammation to reduce cardiovascular disease risk: A realistic clinical prospect? Br. J. Pharmacol.2017, 174, 3898–3913. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O. Multiple Roles for Neutrophils in Atherosclerosis. Circ. Res. 2012, 110, 875. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The Role of Platelet-Activating Factor in Chronic Inflammation, Immune Activation, and Comorbidities Associated with HIV Infection. AIDS Rev. 2015, 17, 191–201. [Google Scholar] [PubMed]
- Demopoulos, C.; Pinckard, R.; Hanahan, D.J. Platelet-activating factor. Evidence for 1-O-alkyl-2-acetyl-sn-glyceryl-3-phosphorylcholine as the active component (a new class of lipid chemical mediators). J. Biol. Chem. 1979, 254, 9355–9358. [Google Scholar] [PubMed]
- Ishii, S.; Nagase, T.; Shimizu, T. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 2002, 68–69, 599–609. [Google Scholar] [CrossRef]
- Honda, Z.; Ishii, S.; Shimizu, T. Platelet-activating factor receptor. J. Biochem.2002, 131, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Palur Ramakrishnan, A.V.K.; Varghese, T.P.; Vanapalli, S.; Nair, N.K.; Mingate, M.D. Platelet activating factor: A potential biomarker in acute coronary syndrome? Cardiovasc. Ther. 2017, 35, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.D.; Navab, M.; Hama, S.Y.; Sevanian, A.; Prescott, S.M.; Stafforini, D.M.; McIntyre, T.M.; Du, B.N.; Fogelman, A.M.; Berliner, J.A. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J. Clin. Investig. 1995, 95, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, W.; Laird, J.; Hazen, S.L.; Salomon, R.G. Polyunsaturated phospholipids promote the oxidation and fragmentation of gamma-hydroxyalkenals: Formation and reactions of oxidatively truncated ether phospholipids. J. Lipid Res. 2008, 49, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Venable, M.E.; Zimmerman, G.A.; McIntyre, T.M.; Prescott, S.M. Platelet-activating factor: A phospholipid autacoid with diverse actions. J. Lipid Res.1993, 34, 691–702. [Google Scholar] [PubMed]
- Damiani, E.; Ullrich, S.E. Understanding the connection between platelet-activating factor, a UV-induced lipid mediator of inflammation, immune suppression and skin cancer. Prog. Lipid Res. 2016, 63, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Zhu, W.; Chen, G.; Luo, X.; Liew, O.W.; Puah, C.M.; Chen, K.; Jiang, H. Understanding the regulation mechanisms of PAF receptor by agonists and antagonists: Molecular modeling and molecular dynamics simulation studies. Proteins 2007, 67, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.D.; Harris, C.S.; Carswell, C.L.; Baenziger, J.E.; Bennett, S.A. Heterogeneity in the sn-1 carbon chain of platelet-activating factor glycerophospholipids determines pro- or anti-apoptotic signaling in primary neurons. J. Lipid Res. 2008, 49, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, T.M. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: Formation, targets, and inactivation. Biochim. Biophys. Acta 2012, 1818, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Alloatti, G.; Camussi, G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol. Rev. 2000, 80, 1669–1699. [Google Scholar] [CrossRef] [PubMed]
- Castro Faria Neto, H.C.; Stafforini, D.M.; Prescott, S.M.; Zimmerman, G.A. Regulating inflammation through the anti-inflammatory enzyme platelet-activating factor-acetylhydrolase. Mem. Inst. Oswaldo Cruz 2005, 100, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Schleimer, R.; Warner, J.; Chilton, F. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J. Immunol. 1991, 147, 660–666. [Google Scholar] [PubMed]
- Francescangeli, E.; Freysz, L.; Goracci, G. PAF-Synthesizing Enzymes in Neural Cells during Differentiation and in Gerbil Brain during Ischemia. In Platelet-Activating Factor and Related Lipid Mediators 2; Springer: Berlin/Heidelberg, Germany, 1996; pp. 21–27. [Google Scholar]
- Tarui, M.; Shindou, H.; Kumagai, K.; Morimoto, R.; Harayama, T.; Hashidate, T.; Kojima, H.; Okabe, T.; Nagano, T.; Nagase, T.; et al. Selective inhibitors of a PAF biosynthetic enzyme lysophosphatidylcholine acyltransferase 2. J. Lipid Res. 2014, 55, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Eto, M.; Shimizu, T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013, 154, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Kotroyiannis, I.; Vassiliadou, C.; Panagiotakos, D.B.; Chrysohoou, C.; Pitsavos, C.; Stefanadis, C. Platelet activating factor (PAF) and activity of its biosynthetic and catabolic enzymes in blood and leukocytes of male patients with newly diagnosed heart failure. Clin. Biochem. 2009, 42, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Marathe, G.K.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Activation of vascular cells by PAF-like lipids in oxidized LDL. Vasc. Pharmacol. 2002, 38, 193–200. [Google Scholar] [CrossRef]
- Marathe, G.K.; Prescott, S.M.; Zimmerman, G.A.; McIntyre, T.M. Oxidized LDL Contains Inflammatory PAF-Like Phospholipids. Trends Cardiovasc. Med. 2001, 11, 139–142. [Google Scholar] [CrossRef]
- Stafforini, D.M. Biology of Platelet-activating Factor Acetylhydrolase (PAF-AH, Lipoprotein Associated Phospholipase A2). Cardiovasc. Drugs Ther. 2009, 23, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, K.; Inoue, K. Overview of PAF-degrading enzymes. The Enzymes2015, 38, 1–22. [Google Scholar] [PubMed]
- Stafforini, D.M.; Zimmerman, G.A. Unraveling the PAF-AH/Lp-PLA(2) controversy. J. Lipid Res. 2014, 55, 1811–1814. [Google Scholar] [CrossRef] [PubMed]
- Tellis, C.C.; Tselepis, A. Pathophysiological role and clinical significance of lipoprotein-associated phospholipase A2 (Lp-PLA2) bound to LDL and HDL. Curr. Pharm. Des. 2014, 20, 6256–6269. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Arai, H. Intracellular PAF-acetylhydrolase type I. The Enzymes 2015, 38, 23–35. [Google Scholar] [PubMed]
- Kono, N.; Arai, H. Intracellular platelet-activating factor acetylhydrolase, type II: A unique cellular phospholipase A2 that hydrolyzes oxidatively modified phospholipids. The Enzymes 2015, 38, 43–54. [Google Scholar] [PubMed]
- Mazereeuw, G.; Herrmann, N.; Bennett, S.A.; Swardfager, W.; Xu, H.; Valenzuela, N.; Fai, S.; Lanctot, K.L. Platelet activating factors in depression and coronary artery disease: A potential biomarker related to inflammatory mechanisms and neurodegeneration. Neurosci. Biobehav. Rev. 2013, 37, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, V.; Bar-Eli, M. Inflammation and melanoma growth and metastasis: The role of platelet-activating factor (PAF) and its receptor. Cancer Metastasis Rev. 2007, 26, 359. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; de Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.Y.; Granger, D.N. Platelets: A critical link between inflammation and microvascular dysfunction. J. Physiol. 2012, 590, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Ed Rainger, G.; Chimen, M.; Harrison, M.J.; Yates, C.M.; Harrison, P.; Watson, S.P.; Lordkipanidzé, M.; Nash, G.B. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets 2015, 26, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Sneddon, A.A.; McLeod, E.; Wahle, K.W.J.; Arthur, J.R. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: Suppression by conjugated linoleic acid. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2006, 1761, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, Z.S.; Jackson, S.P. The Role of Platelets in Atherothrombosis. ASH Educ. Program Book 2011, 2011, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, M.; Hosseini, E. Intravascular leukocyte migration through platelet thrombi: Directing leukocytes to sites of vascular injury. Thromb. Haemost. 2015, 113, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Franks, Z.G.; Campbell, R.A.; Weyrich, A.S.; Rondina, M.T. Platelet–leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann. N. Y. Acad. Sci. 2010, 1207, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Sager, H.B.; Nahrendorf, M. Inflammation: A trigger for acute coronary syndrome. Q. J. Nucl. Med. Mol. Imaging 2016, 60, 185–193. [Google Scholar] [PubMed]
- Cochain, C.; Zernecke, A. Macrophages in vascular inflammation and atherosclerosis. Pflügers Archiv Eur. J. Physiol. 2017, 469, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Gui, T.; Shimokado, A.; Sun, Y.; Akasaka, T.; Muragaki, Y. Diverse Roles of Macrophages in Atherosclerosis: From Inflammatory Biology to Biomarker Discovery. Mediat. Inflamm. 2012, 2012, 693083. [Google Scholar] [CrossRef] [PubMed]
- Rios, F.J.O.; Gidlund, M.; Jancar, S. Pivotal Role for Platelet-Activating Factor Receptor in CD36 Expression and oxLDL Uptake by Human Monocytes/Macrophages. Cell. Physiol. Biochem. 2011, 27, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Sumita, C.; Yamane, M.; Matsuda, T.; Maeda, M.; Nariai, T.; Fujio, Y.; Azuma, J. Platelet activating factor induces cytoskeletal reorganization through Rho family pathway in THP-1 macrophages. FEBS Lett. 2005, 579, 4038–4042. [Google Scholar] [CrossRef] [PubMed]
- Angeli, V.; Llodrá, J.; Rong, J.X.; Satoh, K.; Ishii, S.; Shimizu, T.; Fisher, E.A.; Randolph, G.J. Dyslipidemia Associated with Atherosclerotic Disease Systemically Alters Dendritic Cell Mobilization. Immunity 2004, 21, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.M.; Bizzarro, B.; Sá-Nunes, A.; Rios, F.J.O.; Jancar, S. Activation of PAF-receptor induces regulatory dendritic cells through PGE2 and IL-10. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Li, N. CD4+ T cells in atherosclerosis: Regulation by platelets. Thromb. Haemost.2013, 109, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Murata, T.; Tanaka, H.; Matsuoka, T.; Sakata, D.; Yoshida, N.; Katagiri, K.; Kinashi, T.; Tanaka, T.; Miyasaka, M.; et al. Thromboxane A2 modulates interaction of dendritic cells and T cells and regulates acquired immunity. Nat. Immunol. 2003, 4, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.M.; Zimmerman, G.A.; Stafforini, D.M.; McIntyre, T.M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 2000, 69, 419–445. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J.; Huang, Y.H.; Rönnelid, J.; Schäfer-Elinder, L. Platelet-Activating Factor and Oxidized LDL Induce Immune Activation by a Common Mechanism. Arterioscl. Thromb. Vasc. Biol. 1997, 17, 963. [Google Scholar] [CrossRef] [PubMed]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. Platelet activating factor (PAF). A review of its effects, antagonists and possible future clinical implications (Part I). Drugs 1991, 42, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Koltai, M.; Hosford, D.; Guinot, P.; Esanu, A.; Braquet, P. PAF. A review of its effects, antagonists and possible future clinical implications (Part II). Drugs1991, 42, 174–204. [Google Scholar] [CrossRef] [PubMed]
- Negro Alvarez, J.M.; Miralles Lopez, J.C.; Ortiz Martinez, J.L.; Abellan Aleman, A.; Rubio del Barrio, R. Platelet-activating factor antagonists. Allergol. Immunopathol. 1997, 25, 249–258. [Google Scholar]
- Singh, P.; Singh, I.N.; Mondal, S.C.; Singh, L.; Garg, V.K. Platelet-activating factor (PAF)-antagonists of natural origin. Fitoterapia 2013, 84, 180–201. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, G.; Rabinovici, R.; Leor, J.; Winkler, J.D.; Vonhof, S. Platelet-activating factor and cardiac diseases: Therapeutic potential for PAF inhibitors. J. Lipid Mediat. Cell Signal. 1997, 15, 255–284. [Google Scholar] [CrossRef]
- Loucks, E.B.; Symersky, P.; Qayumi, A.K. Platelet-activating factor antagonism: A new concept in the management of regional myocardial ischemia-reperfusion injury. J. Investig. Surg. 1997, 10, 321–338. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on Platelet Activating Factor Inhibitors: Could a New Class of Potent Metal-Based Anti-Inflammatory Drugs Induce Anticancer Properties? Bioinorg. Chem. Appl. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Choleva, M.; Antonopoulou, S.; Demopoulos, C.A. Wine and its metabolic effects. A comprehensive review of Clinical Trials. Metabolism2018. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Demopoulos, C.A.; Zabetakis, I. In vivo anti-atherogenic properties of cultured gilthead sea bream (Sparus aurata) polar lipid extracts in hypercholesterolaemic rabbits. Food Chem. 2010, 120, 831–836. [Google Scholar] [CrossRef]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Atherosclerosis regression study in rabbits upon olive pomace polar lipid extract administration. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Tsantila, N.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic and antiatherosclerotic properties of olive oil and olive pomace polar extracts in rabbits. Mediat. Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.C.; Antonopoulou, S.; Perrea, D.N.; Sokolis, D.P.; Theocharis, S.E.; Kavantzas, N.; Iliopoulos, D.G.; Demopoulos, C.A. In vivo antiatherogenic properties of olive oil and its constituent lipid classes in hyperlipidemic rabbits. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Argyrou, C.; Vlachogianni, I.; Stamatakis, G.; Demopoulos, C.A.; Antonopoulou, S.; Fragopoulou, E. Postprandial effects of wine consumption on Platelet Activating Factor metabolic enzymes. Prostaglandins Other Lipid Mediat. 2017. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, M.N.; Kalathara, K.; Melachroinou, S.; Arampatzi-Menenakou, K.; Antonopoulou, S.; Yannakoulia, M.; Fragopoulou, E. Wine consumption reduced postprandial platelet sensitivity against platelet activating factor in healthy men. Eur. J. Nutr. 2017, 56, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, M.N.; Asimakopoulos, D.; Antonopoulou, S.; Demopoulos, C.A.; Fragopoulou, E. Effect of Robola and Cabernet Sauvignon extracts on platelet activating factor enzymes activity on U937 cells. Food Chem. 2014, 165, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. In Proceedings of the 45th International Conference on the Bioscience of Lipids, University of Ioannina, Ioannina, Greece, 25–29 May 2004; p. 66. [Google Scholar]
- Fragopoulou, E.; Nomikos, T.; Tsantila, N.; Mitropoulou, A.; Zabetakis, I.; Demopoulos, C.A. Biological activity of total lipids from red and white wine/must. J. Agric. Food Chem. 2001, 49, 5186–5193. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Nomikos, T.; Antonopoulou, S.; Mitsopoulou, C.A.; Demopoulos, C.A. Separation of Biologically Active Lipids from Red Wine. J. Agric. Food. Chem. 2000, 48, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Panayiotou, A.; Samartzis, D.; Nomikos, T.; Fragopoulou, E.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Lipid fractions with aggregatory and antiaggregatory activity toward platelets in fresh and fried cod (Gadus morhua): correlation with platelet-activating factor and atherogenesis. J. Agric. Food. Chem. 2000, 48, 6372–6379. [Google Scholar] [CrossRef] [PubMed]
- Sioriki, E.; Smith, T.K.; Demopoulos, C.A.; Zabetakis, I. Structure and cardioprotective activities of polar lipids of olive pomace, olive pomace-enriched fish feed and olive pomace fed gilthead sea bream (Sparus aurata). Food Res. Int. 2016, 83, 143–151. [Google Scholar] [CrossRef]
- Sioriki, E.; Nasopoulou, C.; Demopoulos, C.A.; Zabetakis, I. Comparison of sensory and cardioprotective properties of olive-pomace enriched and conventional gilthead sea bream (Sparus aurata): The effect of grilling. J. Aquat. Food Prod. Technol. 2015, 24, 782–795. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Smith, T.; Detopoulou, M.; Tsikrika, C.; Papaharisis, L.; Barkas, D.; Zabetakis, I. Structural elucidation of olive pomace fed sea bass (Dicentrarchus labrax) polar lipids with cardioprotective activities. Food Chem.2014, 145, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Gogaki, V.; Stamatakis, G.; Papaharisis, L.; Demopoulos, C.; Zabetakis, I. Evaluation of the in Vitro Anti-Atherogenic Properties of Lipid Fractions of Olive Pomace, Olive Pomace Enriched Fish Feed and Gilthead Sea Bream (Sparus aurata) Fed with Olive Pomace Enriched Fish Feed. Mar. Drugs2013, 11, 3676. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Stamatakis, G.; Demopoulos, C.A.; Zabetakis, I. Effects of olive pomace and olive pomace oil on growth performance, fatty acid composition and cardio protective properties of gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax). Food Chem. 2011, 129, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Karantonis, H.C.; Andriotis, M.; Demopoulos, C.A.; Zabetakis, I. Antibacterial and anti-PAF activity of lipid extracts from sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem.2008, 111, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.; Zabetakis, I. Comparison of antiatherogenic properties of lipids obtained from wild and cultured sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Food Chem.2007, 100, 560–567. [Google Scholar] [CrossRef]
- Rementzis, J.; Antonopoulou, S.; Argyropoulos, D.; Demopoulos, C.A. Biologically active lipids from S. scombrus. In Platelet-Activating Factor and Related Lipid Mediators 2; Springer: Berlin/Heidelberg, Germany, 1996; pp. 65–72. [Google Scholar]
- Karantonis, H.C.; Antonopoulou, S.; Demopoulos, C.A. Antithrombotic lipid minor constituents from vegetable oils. Comparison between olive oils and others. J. Agric. Food Chem. 2002, 50, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Gogaki, V.; Panagopoulou, E.; Demopoulos, C.; Zabetakis, I. Hen egg yolk lipid fractions with antiatherogenic properties. Anim. Sci. J. 2013, 84, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Poutzalis, S.; Anastasiadou, A.; Nasopoulou, C.; Megalemou, K.; Sioriki, E.; Zabetakis, I. Evaluation of the in vitro anti-atherogenic activities of goat milk and goat dairy products. Dairy Sci. Technol. 2016, 96, 317–327. [Google Scholar] [CrossRef]
- Tsorotioti, S.E.; Nasopoulou, C.; Detopoulou, M.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. In vitro anti-atherogenic properties of traditional Greek cheese lipid fractions. Dairy Sci. Technol. 2014, 94, 269–281. [Google Scholar] [CrossRef]
- Megalemou, K.; Sioriki, E.; Lordan, R.; Dermiki, M.; Nasopoulou, C.; Zabetakis, I. Evaluation of sensory and in vitro anti-thrombotic properties of traditional Greek yogurts derived from different types of milk. Heliyon 2017, 3, e00227. [Google Scholar] [CrossRef] [PubMed]
- Apitz-Castro, R.; Cabrera, S.; Cruz, M.R.; Ledezma, E.; Jain, M.K. Effects of garlic extract and of three pure components isolated from it on human platelet aggregation, arachidonate metabolism, release reaction and platelet ultrastructure. Thromb. Res. 1983, 32, 155–169. [Google Scholar] [CrossRef]
- Violi, F.; Pratico, D.; Ghiselli, A.; Alessandri, C.; Iuliano, L.; Cordova, C.; Balsano, F. Inhibition of cyclooxygenase-independent platelet aggregation by low vitamin E concentration. Atherosclerosis 1990, 82, 247–252. [Google Scholar] [CrossRef]
- Kakishita, E.; Suehiro, A.; Oura, Y.; Nagai, K. Inhibitory effect of vitamin E (alpha-tocopherol) on spontaneous platelet aggregation in whole blood. Thromb. Res. 1990, 60, 489–499. [Google Scholar] [CrossRef]
- Ji, W.; Chen, J.; Mi, Y.; Wang, G.; Xu, X.; Wang, W. Platelet-activating factor receptor activation promotes prostate cancer cell growth, invasion and metastasis via ERK1/2 pathway. Int. J. Oncol. 2016, 49, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Karandish, F.; Mallik, S. Biomarkers and Targeted Therapy in Pancreatic Cancer. Biomarkers Cancer 2016, 8, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Anandi, V.L.; Ashiq, K.A.; Nitheesh, K.; Lahiri, M. Platelet-activating factor promotes motility in breast cancer cells and disrupts non-transformed breast acinar structures. Oncol. Rep. 2016, 35, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Saito Rde, F.; Tortelli, T.C., Jr.; Jacomassi, M.D.; Otake, A.H.; Chammas, R. Emerging targets for combination therapy in melanomas. FEBS Lett. 2015, 589, 3438–3448. [Google Scholar] [CrossRef] [PubMed]
- Jancar, S.; Chammas, R. PAF receptor and tumor growth. Curr. Drug Targets2014, 15, 982–987. [Google Scholar] [PubMed]
- Hackler, P.C.; Reuss, S.; Konger, R.L.; Travers, J.B.; Sahu, R.P. Systemic Platelet-activating Factor Receptor Activation Augments Experimental Lung Tumor Growth and Metastasis. Cancer Growth Metastasis 2014, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, M.; Zhang, X.; Cai, Q.; Zhu, Z.; Jiang, W.; Xu, C. Transactivation of epidermal growth factor receptor through platelet-activating factor/receptor in ovarian cancer cells. J. Exp. Clin. Cancer Res. 2014, 33, 85. [Google Scholar] [CrossRef] [PubMed]
- Shida-Sakazume, T.; Endo-Sakamoto, Y.; Unozawa, M.; Fukumoto, C.; Shimada, K.; Kasamatsu, A.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. Lysophosphatidylcholine acyltransferase1 overexpression promotes oral squamous cell carcinoma progression via enhanced biosynthesis of platelet-activating factor. PLoS ONE 2015, 10, e0120143. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gao, L.; Wang, L.; Tang, G.; He, M.; Yu, Y.; Ni, X.; Sun, Y. Effects of platelet-activating factor and its differential regulation by androgens and steroid hormones in prostate cancers. Br. J. Cancer 2013, 109, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Kispert, S.E.; Marentette, J.O.; McHowat, J. Enhanced breast cancer cell adherence to the lung endothelium via PAF acetylhydrolase inhibition: A potential mechanism for enhanced metastasis in smokers. Am. J. Physiol. Cell Physiol. 2014, 307, C951–C956. [Google Scholar] [CrossRef] [PubMed]
- Kispert, S.; Marentette, J.; McHowat, J. Cigarette smoke induces cell motility via platelet-activating factor accumulation in breast cancer cells: A potential mechanism for metastatic disease. Physiol. Rep. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- da Silva-Junior, I.A.; Dalmaso, B.; Herbster, S.; Lepique, A.P.; Jancar, S. Platelet-Activating Factor Receptor Ligands Protect Tumor Cells from Radiation-Induced Cell Death. Front. Oncol. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; He, Z.; Ke, J.; Li, S.; Wu, X.; Lian, L.; He, X.; He, X.; Hu, J.; Zou, Y.; et al. PAF receptor antagonist Ginkgolide B inhibits tumourigenesis and angiogenesis in colitis-associated cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 432–440. [Google Scholar] [PubMed]
- Morita, K.; Shiraishi, S.; Motoyama, N.; Kitayama, T.; Kanematsu, T.; Uezono, Y.; Dohi, T. Palliation of bone cancer pain by antagonists of platelet-activating factor receptors. PLoS ONE 2014, 9, e91746. [Google Scholar] [CrossRef] [PubMed]
- Semini, G.; Hildmann, A.; von Haefen, C.; Danker, K. Glycosidated phospholipids—A promising group of anti-tumour lipids. Anticancer Agents Med. Chem. 2014, 14, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Camussi, G. Potential role of platelet-activating factor in renal pathophysiology. Kidney Int. 1986, 29, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Iatrou, C.; Demopoulos, C.A. Characterization of acetyl-CoA: Lyso-PAF acetyltransferase of human mesangial cells. Mediat. Inflamm. 2005, 2005, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Novoa, J.M. Potential role of platelet activating factor in acute renal failure. Kidney Int. 1999, 55, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Gomez-Chiarri, M.; Lerma, J.L.; Gonzalez, E.; Egido, J. The role of platelet-activating factor (PAF) in experimental glomerular injury. Lipids 1991, 26, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Iatrou, C.; Antonopoulou, S.; Ruan, X.Z.; Fernando, R.L.; Powis, S.H.; Moorhead, J.F.; Varghese, Z. Platelet-activating factor (PAF) increase intracellular lipid accumulation by increasing both LDL and scavenger receptors in human mesangial cells. J. Lab. Clin. Med. 2006, 147, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Correa-Costa, M.; Andrade-Oliveira, V.; Braga, T.T.; Castoldi, A.; Aguiar, C.F.; Origassa, C.S.; Rodas, A.C.; Hiyane, M.I.; Malheiros, D.M.; Rios, F.J.; et al. Activation of platelet-activating factor receptor exacerbates renal inflammation and promotes fibrosis. Lab. Investig. 2014, 94, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shields, L.B.E.; Gao, Z.; Wang, Y.; Zhang, Y.P.; Chu, T.; Zhu, Q.; Shields, C.B.; Cai, J. Current Understanding of Platelet-Activating Factor Signaling in Central Nervous System Diseases. Mol. Neurobiol. 2017, 54, 5563–5572. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, K.M.; Smith, P.F.; Darlington, C.L. Platelet-activating factor in the CNS. Prog. Neurobiol. 1996, 50, 585–596. [Google Scholar] [CrossRef]
- Tsuda, M.; Tozaki-Saitoh, H.; Inoue, K. Platelet-activating factor and pain. Biol. Pharm. Bull. 2011, 34, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Barlow, C.L.; Ramirez, S.H.; Abood, M.E.; Brailoiu, G.C. Effects of Platelet-Activating Factor on Brain Microvascular Endothelial Cells. Neuroscience 2018, 377, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Logan, A.; Cocks, B.G.; Rochfort, S. Seasonal variation of polar lipid content in bovine milk. Food Chem. 2017, 237, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, K.M.; Darlington, C.L.; Smith, P.F. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog. Neurobiol. 2002, 67, 235–257. [Google Scholar] [CrossRef]
- Tomasiak-Lozowska, M.M.; Klimek, M.; Lis, A.; Moniuszko, M.; Bodzenta-Lukaszyk, A. Markers of anaphylaxis—A systematic review. Adv. Med. Sci.2018, 63, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Triggiani, M.; Patella, V.; Staiano, R.I.; Granata, F.; Marone, G. Allergy and the cardiovascular system. Clin. Exp. Immunol. 2008, 153 (Suppl. 1), 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B. Platelet-activating factor (PAF): A review of its role in asthma and clinical efficacy of PAF antagonists in the disease therapy. Rec. Patents on Inflammation Allergy Drug Discovery 2008, 2, 72–76. [Google Scholar]
- Kasperska-Zajac, A.; Brzoza, Z.; Rogala, B. Platelet activating factor as a mediator and therapeutic approach in bronchial asthma. Inflammation 2008, 31, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Palgan, K.; Bartuzi, Z. Platelet activating factor in allergies. Int. J. Immunopathol. Pharmacol. 2015, 28, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Kulinski, J.M.; Munoz-Cano, R.; Olivera, A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur. J. Pharmacol. 2016, 778, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, V. Role of histamine and platelet-activating factor in allergic rhinitis. J. Physiol. Biochem. 2004, 60, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, M.R.; Varricchi, G.; Seaf, M.; Marone, G.; Levi-Schaffer, F.; Marone, G. Bidirectional Mast Cell-Eosinophil Interactions in Inflammatory Disorders and Cancer. Front. Med. 2017, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Mullol, J.; Bousquet, J.; Bachert, C.; Canonica, W.G.; Gimenez-Arnau, A.; Kowalski, M.L.; Marti-Guadano, E.; Maurer, M.; Picado, C.; Scadding, G.; et al. Rupatadine in allergic rhinitis and chronic urticaria. Allergy 2008, 63 (Suppl. 87), 5–28. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Nunez, V.; Bachert, C.; Mullol, J. Rupatadine: Global safety evaluation in allergic rhinitis and urticaria. Expert Opin. Drug. Saf. 2016, 15, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.; Teixeira, M.M.; Souza, D.G. Opportunities for the development of novel therapies based on host-microbial interactions. Pharmacol. Res. 2016, 112, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Grigg, J. The platelet activating factor receptor: A new anti-infective target in respiratory disease? Thorax 2012, 67, 840–841. [Google Scholar] [CrossRef] [PubMed]
- Chatzovoulos, P.; Tsoupras, A.B.; Samiotaki, M.; Panayotou, G.; Demopoulos, C.A.; Dotsika, E. PAF-metabolic enzymes and PAF-like activity in L. infantum and L. major promastigotes. Eur. J. Inflamm. 2011, 9, 231–239. [Google Scholar] [CrossRef]
- Mathiak, G.; Szewczyk, D.; Abdullah, F.; Ovadia, P.; Rabinovici, R. Platelet-activating factor (PAF) in experimental and clinical sepsis. Shock 1997, 7, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Akinosoglou, K.; Alexopoulos, D. Use of antiplatelet agents in sepsis: A glimpse into the future. Thromb. Res. 2014, 133, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P. Therapeutic options directed against platelet activating factor, eicosanoids and bradykinin in sepsis. J. Antimicrob. Chemother. 1998, 41 (Suppl. A), 81–94. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, B.; Huseyinov, A.; Darcan, S. The role of platelet-activating factor in pathogenesis of type 1 diabetes. Diabetes Care 2005, 28, 980. [Google Scholar] [CrossRef] [PubMed]
- Sfredel, V.; Moţa, M.; Trăilă, A.; Dănoiu, S.; Matcaş, H. Disturbances of the coagulating equilibrium of blood in diabetes mellitus. Rev. Roum. Med. Intern.1999, 37, 251–260. [Google Scholar]
- Kudolo, G.B.; DeFronzo, R.A. Urinary platelet-activating factor excretion is elevated in non-insulin dependent diabetes mellitus. Prostaglandins Other Lipid Mediat. 1999, 57, 87–98. [Google Scholar] [CrossRef]
- Liu, L.R.; Xia, S.H. Role of platelet-activating factor in the pathogenesis of acute pancreatitis. World J. Gastroenterol. 2006, 12, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, S.H.; Chen, H.; Li, X.H. Therapy for acute pancreatitis with platelet-activating factor receptor antagonists. World J. Gastroenterol. 2008, 14, 4735–4738. [Google Scholar] [CrossRef] [PubMed]
- Karidis, N.P.; Kouraklis, G.; Theocharis, S.E. Platelet-activating factor in liver injury: A relational scope. World J. Gastroenterol. 2006, 12, 3695–3706. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.L.; Caplan, M.S. Necrotizing enterocolitis: Pathophysiology, platelet-activating factor, and probiotics. Semin. Pediatr. Surg. 2013, 22, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ewer, A.K. Role of platelet-activating factor in the pathophysiology of necrotizing enterocolitis. Acta Paediatr. Suppl. 2002, 91, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Nitoda, E.; Moschos, M.M.; Mavragani, C.P.; Koutsilieris, M. Ocular actions of platelet-activating factor: Clinical implications. Expert Opin. Ther. Targets 2012, 16, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.T.; Gozal, E.; Roberts, A.M. Platelet-mediated vascular dysfunction during acute lung injury. Arch. Physiol. Biochem. 2012, 118, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.J.; Constantinescu, C.S. Platelet activating factor/platelet activating factor receptor pathway as a potential therapeutic target in autoimmune diseases. Inflamm. Allergy Drug Targets 2009, 8, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Bussolati, B.; Rollino, C.; Mariano, F.; Quarello, F.; Camussi, G. IL-10 stimulates production of platelet-activating factor by monocytes of patients with active systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 2000, 122, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.; Solomon, M.J. Crohn’s disease: In defense of a microvascular aetiology. Int. J. Colorectal Dis. 2002, 17, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Macconi, D.; Riccardi, E.; Boccardo, P.; Zilio, P.; Bertani, T.; Remuzzi, G. Platelet-activating factor receptor blocking reduces proteinuria and improves survival in lupus autoimmune mice. J. Pharmacol. Exp. Ther. 1991, 258, 601–606. [Google Scholar] [PubMed]
- Baldi, E.; Emancipator, S.N.; Hassan, M.O.; Dunn, M.J. Platelet activating factor receptor blockade ameliorates murine systemic lupus erythematosus. Kidney Int. 1990, 38, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Karantonis, H.C.; Fragopoulou, E.; Antonopoulou, S.; Rementzis, J.; Phenekos, C.; Demopoulos, C.A. Effect of fast-food Mediterranean-type diet on type 2 diabetics and healthy human subjects’ platelet aggregation. Diabetes Res. Clin. Pract. 2006, 72, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulou, S.; Fragopoulou, E.; Karantonis, H.C.; Mitsou, E.; Sitara, M.; Rementzis, J.; Mourelatos, A.; Ginis, A.; Phenekos, C. Effect of traditional Greek Mediterranean meals on platelet aggregation in normal subjects and in patients with type 2 diabetes mellitus. J. Med. Food 2006, 9, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Fragopoulou, E.; Detopoulou, P.; Nomikos, T.; Pliakis, E.; Panagiotakos, D.B.; Antonopoulou, S. Mediterranean wild plants reduce postprandial platelet aggregation in patients with metabolic syndrome. Metabolism 2012, 61, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Moss, J.W.; Ramji, D.P. Cytokines: Roles in atherosclerosis disease progression and potential therapeutic targets. Fut. Med. Chem. 2016, 8, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Capra, V.; Bäck, M.; Barbieri, S.S.; Camera, M.; Tremoli, E.; Rovati, G.E. Eicosanoids and Their Drugs in Cardiovascular Diseases: Focus on Atherosclerosis and Stroke. Med. Res. Rev. 2013, 33, 364–438. [Google Scholar] [CrossRef] [PubMed]
- Feige, E.; Mendel, I.; George, J.; Yacov, N.; Harats, D. Modified phospholipids as anti-inflammatory compounds. Curr. Opin. Lipidol. 2010, 21, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Invited review: The anti-inflammatory properties of dairy lipids. J. Dairy Sci. 2017, 100, 4197–4212. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Zabetakis, I. Ovine and caprine lipids promoting cardiovascular health in milk and its derivatives. Adv. Dairy Res. 2017, 5. [Google Scholar] [CrossRef]
- Rizzo, M.; Otvos, J.; Nikolic, D.; Montalto, G.; Toth, P.; Banach, M. Subfractions and subpopulations of HDL: An update. Curr. Med. Chem. 2014, 21, 2881–2891. [Google Scholar] [CrossRef] [PubMed]
- Marathe, G.K.; Pandit, C.; Lakshmikanth, C.L.; Chaithra, V.H.; Jacob, S.P.; D’Souza, C.J.M. To hydrolyze or not to hydrolyze: The dilemma of platelet-activating factor acetylhydrolase. J. Lipid Res. 2014, 55, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Goszcz, K.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Bioactive polyphenols and cardiovascular disease: Chemical antagonists, pharmacological agents or xenobiotics that drive an adaptive response? Br. J. Pharmacol. 2017, 174, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M. Multivitamins in the prevention of cardiovascular disease in men: The physicians’ health study II randomized controlled trial. JAMA 2012, 308, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Lonn, E.; Yusuf, S.; Hoogwerf, B.; Pogue, J.; Yi, Q.; Zinman, B.; Bosch, J.; Dagenais, G.; Mann, J.F.E.; Gerstein, H.C. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: Results of the HOPE study and MICRO-HOPE substudy. Diabetes Care 2002, 25, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- The Heart Outcomes Prevention Evaluation Study Investigators. Vitamin E Supplementation and Cardiovascular Events in High-Risk Patients. NEMJ 2000, 342, 154–160. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Leslie, S.J.; Megson, I.L. Antioxidants in cardiovascular therapy: Panacea or false hope? Front. Cardiovas. Med. 2015, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, L.A.; Khaw, K.T.; Bingham, S.; Day, N.E.; Luben, R.N.; Oakes, S.; Welch, A.; Wareham, N.J. Fruit and vegetable intake and population glycosylated haemoglobin levels: The EPIC-Norfolk Study. Eur. J. Clin. Nutr.2001, 55, 342. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.-K.; Ju, W.; Cho, B.; Oh, S.-W.; Park, S.M.; Koo, B.-K.; Park, B.-J. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. Br. Med. J. 2013, 346. [Google Scholar] [CrossRef] [PubMed]
- Gale, C.R.; Martyn, C.N.; Winter, P.D.; Cooper, C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ 1995, 310, 1563–1566. [Google Scholar] [CrossRef] [PubMed]
- Detopoulou, P.; Demopoulos, C.; Karantonis, H.; Antonopoulou, S. Mediterranean diet and its protective mechanisms against cardiovascular disease: An insight into Platelet Activating Factor (PAF) and diet interplay. Ann. Nutr. Disord. Ther. 2015, 2, 1–10. [Google Scholar]
- Stoner, G.D.; Sardo, C.; Apseloff, G.; Mullet, D.; Wargo, W.; Pound, V.; Singh, A.; Sanders, J.; Aziz, R.; Casto, B.; et al. Pharmacokinetics of Anthocyanins and Ellagic Acid in Healthy Volunteers Fed Freeze-Dried Black Raspberries Daily for 7 Days. J. Clin. Pharmacol. 2005, 45, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

DIET: Can you REALLY eat yourself happy? You really are what you eat – and your brain health and mood are no exception. For example: Junk food really can affect our mental health by causing blood sugar highs and lows. This interferes with brain chemicals that affect mood. Protein is vital to make serotonin and dopamine – which are key to our mental health. There are crucial nutrients that our brain requires in tiny amounts that can really affect our mental state. They include B vitamins, iron, calcium, magnesium, chromium, zinc and selenium. When you feel tired, do you reach for a sweet snack? And when you're upset, do you find solace in chocolate, ice-cream or a bag of chips? Research confirms that you are more likely to turn to junk food when you're in a bad mood, as their high fat and sugar content activates pleasure centres in the brain, triggering the release of 'feel-good' neurotransmitters such as dopamine and serotonin. But there's a catch. Self-medicating with junk food also leads to blood sugar highs and lows that interfere with how these brain chemicals function to regulate mood. The overall effect can worsen your mental health. NOTE:Eating a variety of whole foods, real foods, supply your brain with the essential nutrients that can help produce a sense of well-being and higher moods. Eating smart is not only good for your well-being but for your families as well.

IN THE NEWS: Poor sleep linked to toxic buildup of Alzheimer’s protein, memory loss Sleep may be a missing piece in the Alzheimer’s disease puzzle. UC Berkeley scientists have found compelling evidence that poor sleep — particularly a deficit of the deep, restorative slumber needed to hit the save button on memories — is a channel through which the beta-amyloid protein believed to trigger Alzheimer’s disease attacks the brain’s long-term memory. “Our findings reveal a new pathway through which Alzheimer’s disease may cause memory decline later in life,” said UC Berkeley neuroscience professor Matthew Walker, senior author of the study published today in the journal Nature Neuroscience. A 2013 University of Rochester study found that the brain cells of mice would shrink during non-rapid-eye-movement (non-REM) sleep to make space for cerebrospinal fluids to wash out toxic metabolites such as beta-amyloid. “Sleep is helping wash away toxic proteins at night, preventing them from building up and from potentially destroying brain cells,” Walker said. “It’s providing a power cleanse for the brain.” NOTE: We have tools to help improve the quality of your sleep.

CHIROPRACTIC: Natural healthcare can help you There are many reasons for the shift in healthcare. Some people are concerned about the side effects that drugs have, (2000 deaths per week over 1 million injuries per year), some people have a sense of body ecology and don't want to put any artificial substances within their body. Some people have discovered drugs mainly cover-up symptoms and never get to the root of your problem, and are more concerned with maximizing their health than just making some symptoms disappear. I'm sure you have seen either a popular magazine or news program on TV, the benefits of different natural healthcare techniques. Chiropractic is the world's largest natural healthcare profession and specializes in finding the root cause of health problems and helping restore patients to better health through natural methods. At Keefe clinic these are the principles that we specialize in. We promise you that we will not just cover-up your symptoms and allow the underlying condition time to get worse. Chiropractic care, homeopathic care, individualized diet, acupuncture, nutritional therapy, muscle therapy and exercise, whatever it takes to move your body from disease to health, dysfunction to realizing your genetic potentials. We will customize a program to fit your particular health needs, from low back pain and headaches to digestive problems and PMS. From ear infections in babies to arthritis or heart disease and the geriatric patient, we offer the latest and natural healthcare. For more information visit our website: www.Keefeclinic.com

FUNNY BONE: Borrow money from pessimists -- they don't expect it back.@@ 99% of lawyers give the rest a bad name.@@82.7% of all statistics are made up on the spot.@@A clear conscience is usually the sign of a bad memory.@@All those who believe in psycho kinesis, raise my hand.@@OK, so what's the speed of dark?@@How do you tell when you're out of invisible ink?.@@ What do you get if you divide the circumference of a pumpkin by its diameter? PUMPKIN PI @@ An actor had been out of work for 15 years because he always forgot his lines. Then one day he got a phone call from a director who wanted him for a big part in a play. All he had to say was “Hark! I hear the cannon roar! After much worry the actor decided to take the role. Opening night arrived, and while he waited in the wings, the actor muttered to himself “Hark! I hear the cannon roar! Hark! I hear the cannon roar! The time for the entrance finally came and as the actor made his appearance, he heard a loud brooooom! He turned around and said, “What the heck was that?” -Robert De Nero
Visit our web sites: keefeclinic.com & facebook.com/keefeclinic
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: ‘What the Health’ — Where This Vegan PR Film Went Wrong The documentary, “What the Health,” ignores accumulated evidence against sugar in a misguided effort to promote vegan ideology. According to the film, the focus on sugar as a contributor to obesity, diabetes and ill health has steered people away from the real culprits, which they claim are meat and animal fat. For example, Dr. Neal Barnard, adjunct associate professor of medicine at the George Washington University School of Medicine and Health Sciences and president of the Physicians Committee for Responsible Medicine, claims that diabetes is not caused by a high-carb, high-sugar diet. In his view, diabetes is caused by fat buildup caused by a meat-based diet. To treat diabetes, Barnard recommends a low-fat vegetarian diet, free of any and all animal products, without any restrictions on carbohydrates. The sugar industry promotes the myth that saturated fat is to blame for weight gain and ill health, not sugar, along with the thoroughly debunked energy balance theory. Fortunately, some great books have now been written exposing the history and extent of the cover-ups. Two examples are science journalist Gary Taubes' book, "The Case Against Sugar," and Marion Nestle's "Soda Politics: Taking on Big Soda (and Winning)." While a high-vegetable diet is certainly beneficial, the low-fat, unrestricted-carb recommendations are not. Low-carb, high-fat diets have proven superior for controlling insulin resistance, which is the hallmark of obesity and metabolic dysfunction. NOTE: This article points out the fight within nutritional science between the high protein low carb diet and the high complex carb low-fat diet. The truth is both diets are correct for different populations, 60 to 70% of the population should be on a high protein low carbohydrate diet where 30 to 40% of the population need to be on a low fat high complex carb diet. It’s a matter of genetics, we are not all the same. KNOW your body type!

WELLNESS: Are you getting enough fiber? One of the most underserved nutrients today is fiber. Typically missing or very low in packaged and fast foods, fiber intake for most Americans is woefully low. Adults need between 20 to 30 grams of fiber every day for optimal health, but most Americans get only about 15. I believe the ideal is closer to 32 grams. What potential benefits might you expect when you increase the amount of fiber you eat each day? Fiber: Assists in managing your weight by helping you feel full longer Helps promote regularity Helps support your heart health Helps support healthy GI function. Your body needs two different types of fiber: soluble and insoluble. Soluble fiber helps slow down your digestion and helps maintain glucose and blood cholesterol levels already in the normal range. Good food sources are nuts, seeds, apples, and blueberries. Insoluble fiber helps propel food through your digestive tract and promotes regularity. Good food sources include dark green leafy vegetables, green beans, celery, carrots, and brown rice. Many whole foods, especially fruits and vegetables, contain both soluble and insoluble fiber, a real nutritional bonus.

CHIROPRACTIC: How Does Chiropractic Work? Many people seek chiropractic care when their back goes out or their neck tightens up. But how does this form of care actually work? What are the benefits of receiving chiropractic care for nerve dysfunction compared with other healthcare options? Let’s take a look! First, let’s discuss how the nervous system “works.” We have three divisions of the nervous system: the central, peripheral, and autonomic nervous systems. The central nervous system (CNS) includes the brain and spinal cord, and it’s essentially the main processing portion of the nervous system. The spinal cord is like a multi-lane highway that brings information to the brain for processing (sensory division) and returns information back to the toes, feet, legs, and upper extremities from which the information originated (motor division). For example, hiking on a mountain trail or simply walking requires constant input to and from the CNS so we can adjust our balance accordingly and not fall. These “sensory-motor pathways” are essential and allow us to complete our daily tasks in an efficient, safe manner as information is constantly bouncing back and forth between the brain and the rest of the body. The peripheral nervous system (PNS) includes a similar sensory/motor “two-way street” system relaying information back and forth from our toes/feet/legs and fingers/hands/arms to the spinal cord (CNS). And if this isn’t complicated enough, we also have “reflexes” that, for example, allow us to QUICKLY pull our hand away from a hot stove to minimize burning our fingers. Reflexes allow the information to “skip” the brain’s processing part so quicker reactions can occur. The autonomic nervous system (ANS) includes the sympathetic and parasympathetic divisions that basically “run” our automatic (organ) functions like breathing, heart rate, digestion, hormonal output, and more. There is constant communication between the ANS, PNS, and CNS that allow us to function in a normal, balanced way… unless something disrupts them. There are obvious conditions that interfere with this communication process that include (but are not limited to) diabetes (with neuropathy), frost bitten or burned fingers, peripheral nerve damage from conditions like carpal/cubital tunnel syndromes, thoracic outlet syndrome, and/or pinched nerves in the neck, mid-back, low-back spinal regions, as well as conditions such as multiple sclerosis (MS), Guillain-Barre Syndrome, after a stroke (spinal cord or brain), and after trauma with resulting fractures where nerve, spinal cord, and/or brain damage can occur. These are “obvious” reasons for delayed or blocked neurotransmission. There are many other less obvious injuries or conditions that can result in faulty neuromotor patterns and nerve transmission of which chiropractic services can benefit. The “subluxation complex” is a term some chiropractors use to describe the compromised nerve transmission that may occur if a nerve is compressed or irritated due to faulty bone or joint position along the nerve’s course. Reducing such nerve compression typically allows for a restoration of function. A good illustration of this is a patient who suffers from a herniated disk in the neck with numbness and tingling down the arm to the hand. The goal of treatment (for all healthcare professionals) is to remove the pinch of the nerve. To realize this goal, doctors of chiropractic utilize spinal manipulation and mobilization in addition to other non-surgical, non-drug approaches that may include exercises, nutritional advice, home-care such as a cervical traction unit, and other anti-inflammatory measures (ice, modalities like low level and class IV laser, electric stimulation, pulsed magnetic field, and more). Given the minimal side-effect risks and well-reported benefits, it only makes sense to try chiropractic FIRST and if you’re not satisfied, your doctor will help you find the next level of care.

FUNNY BONE: Ah, marriage. I was standing in front of the bathroom mirror one evening admiring my reflection, when I posed this question to my wife of 30 years: “Will you still love me when I’m old, fat, and balding?” She answered, “I do.”@@ As the music swelled during a recent wedding reception, my hopelessly romantic husband squeezed my hand, leaned in, and said, “You are better looking than half the women here.”
Visit our web site: keefeclinic.com or faceebook/keefeclinic.com

CLICK HERE
CLICK PIC
Dr. John Folts is currently the Director of the Coronary Thrombosis Research Laboratory at The University of Wisconsin in Madison, Wisconsin.

CLICK PIC
Dr. Folts’ current research is on in vitro platelet mediated coronary thrombosis and anti-oxidants. Dr. Folts developed the experimental model on Aspirin in 1974 and demonstrated by in vitro testing that aspirin can reduce arterial blood clots.
The anti-stick factor

CLICK HERE
Platelets, a class of blood cells, aid in clotting. They keep a fresh cut in the skin from causing life-threatening blood loss. In persons with coronary artery disease, however, overly sensitive platelets may foster clots that obstruct blood flow through vessels already narrowed by fatty deposits. The result can be a heart attack.

CLICK HERE
In a host of studies, Folts' team has shown that red wine and dark beers inhibit platelets in the blood from becoming unusually sticky and generating those risky, clot-forming clumps. Indeed, these dark-colored drinks clearly outperform lighter ones, such as vodka, white wine, and pale lagers.

CLICK HERE
What tends to distinguish a dark beer from a light brew, or a ruby-hued wine from a white one, is their concentrations of quercetin, rutin, resveratrol, and other flavonoid pigments.

CLICK HERE
In their new study, the Wisconsin scientists initially recruited five healthy men and women (including Folts) to drink about three servings of purple grape juice at a sitting -- some 20 to 24 ounces. An hour later, the researchers sampled the volunteers' blood and put it into a device that measures platelet stickiness. Compared to blood samples taken an hour prior to drinking the juice, the later blood was almost 50 percent less prone to platelet clumping.
That's a lot of juice, Folts acknowledges, so he decided to explore what would happen if he halved the dose and drank it daily for a week. Two or 3 days after the last glass was drunk, each volunteer returned for another blood test.

CLICK HERE
The results, reported this week, indicate that regular consumption of the popular juice appears to have a carryover effect. Even after a hiatus of several days, the volunteers' blood was about 25 percent less likely to clump than before the treatment began.
 This finding was particularly welcome, Folts notes, because most heart attacks occur in the early morning, sometimes just as an individual is getting up. His group's data now suggest that even if the juice had been drunk the day before, it would still confer some protection.
This finding was particularly welcome, Folts notes, because most heart attacks occur in the early morning, sometimes just as an individual is getting up. His group's data now suggest that even if the juice had been drunk the day before, it would still confer some protection.
For nearly a decade, cardiologists have known that healthy men over 50 -- and postmenopausal women -- can nearly halve their risk of heart attack by taking an aspirin every other day. Like grape juice, this drug appears to work by inhibiting platelet aggregation.

CLICK HERE
However, when adrenaline is pumped into the blood in response to stress or heavy exercise, Folts points out, aspirin's platelet inhibition virtually disappears. So in the new study, he tested grape juice drinkers' blood to see if the same thing happened to them.
In one case, he took the volunteers' blood, added adrenaline to it, and then measured its susceptibility to platelet clumping. In another, some volunteers exercised and then provided blood for analysis. In both cases, Folts says, the juice depressed platelet clumping.

CLICK HERE
In the late seventies however, we discovered that if we raised the adrenaline or epinephrine, that the same thing, 75% -80% of the time clot formation comes back with aspirin and that’s a serious problem. That’s why we have spent the last 20 years looking for drugs and food substances that would inhibit the platelets and prevent the clot formation, but also, prevent the renewal of clot formation from elevated epinephrine.

CLICK HERE
Now, in our previous findings with the animal model, we have shown that red wine, moderate amounts equivalent to 2 glasses in you or I, purple grape juice; not the clear or white grape juice, tea, but not coffee, in this animal model reduced or inhibited platelet mediated clot formation and protected against potentially fatal coronary thrombosis. These flavonoids in red wine, purple grape juice and tea inhibit platelets about the same as aspirin but they have one unique feature. THEY PROTECT AGAINST ELEVATIONS OF ADRENALINE, WHICH ASPIRIN DOES NOT. That makes the flavonoids more desirable.

Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

DIET: Don’t believe the American Heart Assn. — butter, steak and coconut oil aren’t likely to kill you Last month, the American Heart Assn. once more went after hamburger, beef and especially coconut oil with this recognizable warning: The saturated fats in these foods cause cardiovascular disease. The company’s “presidential advisory” has been a brand new look in the science and arrived in reaction to a growing number of researchers, such as myself, that have pored over the exact information in the past several decades and beg to disagree. A review of the evidence indicates that in regards to mortality or heart attacks fats aren’t guilty. It had been in 1961 that the AHA found the world’s very first official recommendations to prevent saturated fats, together with dietary cholesterol, to be able to protect against a heart attack. This “diet-heart theory” was embraced by the majority of major specialists, though it hadn’t been examined in clinical trials — the only sort of science which may establish cause-and-effect. From the start, the rap on fats lacked a scientific basis. The diet-heart theory was examined more than any other in the history of nourishment, and so far, the results are null. The theory had some funds from preliminary information, plus it made intuitive sense — fat clogs your arteries such as warm grease down a chilly drain tube, right? — that sufficient for AHA officials sought to deal with the wave of cardiovascular disease. Nevertheless, rigorous information was required, and so authorities across the world — such as our very own, during the National Institutes of Health — spent countless dollars attempting to demonstrate the theory was true. Somewhere between 10,000 and 53,000 folks have been analyzed on diets in which saturated fats have been replaced by unsaturated vegetable oils. As anticipated, the results didn’t turn out — people weren’t being killed by saturated fats. Note from Dr. Keefe: there hasn’t been one successful trial showing that fat causes heart disease and death in the last 50 years.

IN THE NEWS: Heart surgeon speaks out on what really causes heart disease Dr. Dwight Lundell We physicians with all our training, knowledge and authority often acquire a rather large ego that tends to make it difficult to admit we are wrong. So, here it is. I freely admit to being wrong. As a heart surgeon with 25 years experience, having performed over 5,000 open-heart surgeries, today is my day to right the wrong with medical and scientific fact. I trained for many years with other prominent physicians labelled “opinion makers.” Bombarded with scientific literature, continually attending education seminars, we opinion makers insisted heart disease resulted from the simple fact of elevated blood cholesterol. The only accepted therapy was prescribing medications to lower cholesterol and a diet that severely restricted fat intake. The latter of course we insisted would lower cholesterol and heart disease. Deviations from these recommendations were considered heresy and could quite possibly result in malpractice. It Is Not Working! Read his complete letter At keefeclinic.com at the following address: http://www.keefeclinic.com/wp/heart-problems-do-the-experts-know-what-they-are-doing/
CONDITION OF THE WEEK: Is It My Low Back Or My Hip? When patients present with low back pain, it is not uncommon for pain to arise from areas other than the low back, such as the hip. There are many tissues in the low back and hip region that are susceptible to injury with have overlapping pain pathways that often make it challenging to isolate the truly injured area. Hip pain can present in many different ways. When considering the anatomy of the low back (lumbar spine) and hip, and the nerves that innervate the hip come from the low back, it’s no wonder that differentiating between the two conditions is often difficult. To make diagnosis even more complex, the hip pain patient may present one day with what appears to be sciatic nerve pain (that is, pain shooting down the back of the leg to the knee if mild or to the foot if more severe) but the next visit, with only groin pain. When pain radiates down a leg, the almost automatic impression by both the patient and their healthcare provider is, “…it’s a pinched nerve.” But again, it could be the hip and NOT a pinched nerve that is creating the leg pain pattern. Knee & ankle reflexes and sensation are normal but muscle strength may be weak due to pain. Bending the low back into different positions does not reproduce pain if the pain is only coming from the hip. Though sometimes challenging, doctors of chiropractic are well-trained to be able to differentiate between hip and low back pain and will treat both areas when it is appropriate.

FUNNY BONE: From the BBC-by John Cleese ANNOUNCEMENT The English are feeling the pinch in relation to the recent terrorist threats and have therefore raised their security level from "miffed" to "peeved." Soon, though, security levels may be raised yet again to "irritated" or even "a bit Cross." The English have not been "a bit Cross" since the blitz in 1940 when tea supplies nearly ran out. The terrorist have been recategorized from "tiresome" to "a bloodied nuisance." The last time the British issued a "bloodied nuisance" warning level was and 1588, when threatened by the Spanish Armada. The Scots have raised their threat level from "pissed off" to "let's get the bastards." They don't have any other levels this is the reason they been used on the front lines of the British Army for the past 300 years.

Visit our web sites: keefeclinic.com & facebook.com/keefeclinic
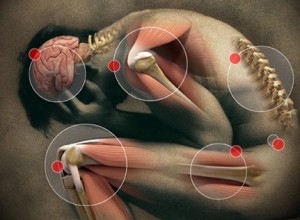
The last thing you might expect going to a doctor for a health problem is becoming a drug addict in the process. In the U.S., painkiller addiction is so rampant that 91 Americans die every day from an overdose of prescription opioids or heroin.

In 1986 the government sent a report to every doctor and healthcare facility in the US. That report was based on extensive research into back pain. Most people might not realize that low back pain is the number 1 health problem in the United States. It's responsible for more time off work, the high cost of rehabilitation on top of the extra high cost of surgery. Since surgery is only 20 to 30% effective and cost a lot of money the government decided to find the most effective treatment for back pain. After they evaluated all the research from around the world the number 1 active treatment they recommended was spinal adjustments. And so they sent this report to every healthcare facility in the United States in hopes of saving the country money.

Unfortunately this report was largely ignored. Surgeons kept doing ineffective surgery, and drug therapist kept giving more powerful painkillers. Today if you listen to news reports you realize we are having an opiate addiction problem like none other. Even though opiates can block pain they also cause a lot of drug addicts to this deadly drug. Since our healthcare system seems to focus more on gaining power over helping patients then you have to be your own protector. Just because a doctor writes your prescription doesn't mean that prescription is going to be both safe and effective for you.

Studies have shown over the past 50 years or so that there are over 2000 people who die every week from prescriptions they get from their doctor. These statistics only come from hospital bound patients we’re not for sure how many a week die who are not hospitalized. 2000 a week is like 3 jumbo jets crashing every week with no survivors for the past 50 years. There are over a million hospitalizations related to drug therapy per year. Now I would be the 1st to say that in certain conditions drugs are necessary to keep a person alive i.e. insulin for type 1diabetes. But the percent of the population who needs prescriptions to survive is a very low percent. Most people's conditions could be safely and effectively resolved through natural healthcare and chiropractic.
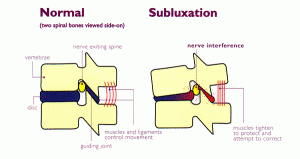
A seven-year study showed that patients whose primary physician was a chiropractor, experienced these results: 60% less hospital admissions, 59% less days in the hospital, 62% less outpatient surgeries, 85% less in pharmaceutical costs. Not only is natural healthcare/chiropractic substantially more effective in the countries’ number 1 health problem (low back pain) nutritional therapy and chiropractic can effectively resolve most nonemergency health challenges.
If you're having a heart attack you need to be in the hospital but after you’re released, if you want to fix the underlining problem that led to the heart attack, then drugs are not going to help you very much. Natural healthcare/chiropractic focuses on the underlying cause of disease. For instance type II diabetes is a growing problem in our society and drugs do not correct that, insulin might be essential in type I diabetes for those bodies who can't make insulin so you have to take it in order to stay alive. But conditions like type II diabetes are related to dietary faults and the subsequent over stressing of the bodies sugar management systems. Simple dietary changes along with targeted nutritional therapy can help the cells recognize insulin and self-regulate blood sugar levels.

I don't care if we are talking about allergies, blood pressure, autoimmune problems, and hormone problems in a high percent of cases you simply find the cause and correct the cause and health is the result. But we live in a pharmaceutical controlled society when it comes to healthcare. Multibillion dollar pharmaceutical companies have taken over healthcare and most clinics and hospitals. Unfortunately their bottom line is not your health but their bank account.

Just because you can take a drug and that drug may make acid reflux feel better doesn't mean that's the correct approach. Shutting down your digestive system at the level of the stomach will deftly remove acid that might burn your esophagus but it will also remove the initial digestive process to properly digest and absorb nutrients from what you eat. That's why studies of people who have taken antacids for long periods of time develop signs and symptoms of malnutrition. When you mess with the digestive system you mess with life. Acid reflux like headaches or back pain have a cause. Never is that because of a lack of pharmaceuticals.
Unfortunately in today's healthcare system symptom relief has become the dominant approach. That makes as much sense as clipping the wire to the warning light on your dashboard and thinking you've taken care of something, yes the warning light has gone off but the problem still there. This is one of the reasons why chronic disease has skyrocketed over the past 40 years. Who would guess it? If you don't fix the problem it just becomes much worse.
Natural healthcare/chiropractic offers a completely different approach to health care in that we don't play the symptom relief came. Our total focus is on finding the cause and then developing effective strategies to resolve the cause. When the cause is eliminated so are the symptoms.

You might think all doctors have this mindset but not if their primary treatment is pharmaceuticals. Except in very rare cases pharmaceuticals are never the appropriate treatment for most healthcare problems. If you have reoccurring headaches then either you have vertebra in your neck out of place or you have toxicity issues or possibly hormonal issues that need to be corrected. Painkillers don't fix any of those possible causes. Painkillers are the biggest killers in drug therapy. Several well-known painkillers have been taken off the market over the past 20 years because of the death rate. And opiates will not only kill you but they can cause a lifetime addiction and they don't fix at all the underlying cause for pain.
Buyer beware is a important slogan to protect yourself in the marketplace. Unfortunately in today's healthcare system you need to take responsibility for yours and your families choices in healthcare.
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: The science behind why chocolate and peanut butter make an irresistible match Raise your hand if you've ever wolfed down several mini chocolate peanut butter cups in a drug-like trance. One bite and you're smitten — the chocolate hits your taste buds and your brain's pleasure centers light up like a Christmas tree. You can't stop yourself from eating more and more peanut butter cups to make the feeling last a little longer. Chocolate and peanut butter are powerful partners in crime that can hijack willpower — fast. From Reese's candies to Tagalong Girl Scout cookies to buckeyes, some of the most beloved candies feature the two flavor co-conspirators. Together, chocolate and peanut butter are somehow even more magnificent than they are when eaten solo. All of this made us wonder — why do chocolate and peanut butter taste so dang heavenly when mixed together? Here's what we found out. Here's why you're obsessed with chocolate and peanut butter The pleasing taste of biting into a Reese's Peanut Butter Cup is partially thanks to a phenomenon called "dynamic sensory contrast," Gregory Ziegler, a Penn State University professor of food science who studies chocolate, said in a phone interview. Ziegler explained that our taste buds love when foods have contrasting textures. "The smooth melt of the chocolate and the crunch of the nut pieces" provide a pleasing contrast, Ziegler noted. The sensual sensation sometimes causes us to eat more — so you can thank dynamic sensory contrast the next time you inhale a dozen mini peanut butter cups in one sitting. Dynamic sensory contrast is also known as the "ice cream effect," Ziegler said. "The idea was, why do people still have room for ice cream [after a huge dinner]?" Even when people report they are extremely full, they often manage to make room for ice cream because our taste buds crave that sensation — when cold ice cream changes its texture as it hits the warmth of your mouth, Ziegler noted. Similarly, chocolate also changes texture (by melting) when it's warmed by your mouth, which is why even chocolate without nuts or peanut butter can be irresistible to our taste buds, Ziegler said.

WELLNESS: Taking high dosages of statins raises the risk of developing diabetes in older women by 50% Taking statins can increase the risk of developing diabetes for older women by more than 50 per cent, research shows. Higher dosages mean they are likelier to suffer the potentially fatal condition – in which blood sugar levels get too high. The controversial cholesterol-lowering drugs are used by around 12million Britons, but have been linked with side effects. Now, Australian scientists have carried out one of the first studies of its kind focusing on the effects of statins on more than 8,000 female pensioners. Taking statins can increase the risk of developing diabetes for older women by more than 50 per cent, research shows. Note: Medical practice concerning cholesterol was based on a fraudulent study over 50 years ago. Even though no one has been able to confirm that study after several tries the train has already left the station. It’s very difficult to change medical practice that’s been going on for many years. Even though there have been no successful cholesterol studies statins are still commonly prescribed for a condition that is caused by inflammation. Elevated cholesterol is a nutritional problem and needs to be addressed through diet, nutrition and lifestyle measures. Don’t risk your health taking dangerous and ill prescribed drugs seek solutions for your health problems.

CONDITION OF THE WEEK: Disc problems: chiropractic versus steroid injections. The recent study comparing the effectiveness of chiropractic to a common practice of steroid injections from medical professionals have found chiropractic adjustments superior. Not only do the adjustments produce better outcome but there is significantly less side effects. What the study doesn’t cover is the difference between correcting the problem and suppressing the problem with drugs. When you have a disc problem you have an alignment problem in virtually all cases. Drugs cannot realign the spine. Even if the drug gave you symptomatic improvement the underlying issue is still present and disc damage is still progressing. When you correct the problem with adjustments, as you realign the vertebra you re-stabilize the disc which allows for normal healing. The difference in both approaches would not be fully realized until at least 5 to 10 years. At that point the continued deterioration would become obvious again under the drug therapy whereas the chiropractic approach, by fixing the problem, offers a better long-term outcome.

FUNNY BONES: I wonder what Facebook employees do to waste their time at work?@@ Someone figured out my password. Now I have to rename my dog.@@ Anton, do you think I’m a bad mother? My name is Paul.@@ It’s ok to talk to yourself, it’s even ok to answer yourself.. But when you ask yourself to repeat what you just said- you have a problem!@@ Dear Google: They are only using you to get to me. Sincerely Wikipedia@@ Whatever you do always give 100% ….. Unless you are donating blood 🙂
Visit our web site: keefeclinic.com&facebook.com/keefeclinic
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: What is alkaline water and can it really help with heartburn? You may have noticed the buzz about alkaline water recently: It’s a staple of the alkaline diet, and promoted by celebs like Miranda Kerr for its supposed health perks. Among those perks is the belief that alkaline H2O—which, by definition, has a higher pH (and lower acidity) than what comes out of the tap—can help neutralize stomach acid and relieve symptoms of gastroesophageal reflux, aka heartburn. NOTE: though this article was interesting and it does show benefits for heartburn that’s not the primary purpose of alkaline water. Alkaline water is one tool along with other foods and drinks to change your pH to more normal setting. Both urine and saliva should be 6.4. When your urine pH gets down to 5.5 or below this puts your body under stress. One of the things that happens is you start leeching alkaline minerals from your bone and teeth. So alkaline water can be an important tool if you system is hyper acidic which usually means it’s inflamed.

WELLNESS: The secret to surviving a heart attack? Eating plenty of salmon, pecans and vegetable oils 'lowers chances of dying' Oily fish like mackerel, salmon and sardines have high levels of omega-3, Some nuts, seeds and oils contain high levels of plant-based equivalent, Study looked at research based on more than 45,000 patients globally. These foods reduce inflammation in the body which is the latest scientific understanding for heart disease. The cholesterol theory for heart disease has never once been proven through scientific studies in the past 50 years. Unfortunately sometimes politics and fraud produce practices that takes years to expose and change. If you’re taking drugs for cholesterol your shortening your life span. Change your diet and lifestyle and you can substantially reduce your likelihood of having a heart attack. Note: Ask for list of anti-inflammatory foods.
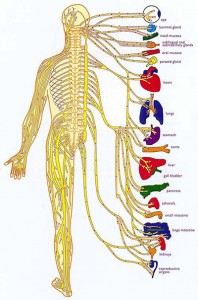
CHIROPRACTIC: The neurological bases for neck and arm symptoms Your neck contains the smallest vertebrae in your spine with the least amount of support. Neck problems can arise from simple headdown posture to whiplash type injuries. When these bones lose their proper position and balance they can stress the delicate nerves that travel into the muscles and tissues of the neck and on down the arm to the tip of the fingers. This can be the source of neck pain, stiffness, limited rotation and problems sleeping. These nerves also innervate the shoulder and can lead to different types of shoulder dysfunction, the elbow leading to tennis elbow type symptoms, the wrist leading to carpal tunnel syndrome and into the fingers leading to grip strength issues, numbness and tingling within the hand. Medications, of course, do not realign the spine. And suppressing symptoms thus allowing misalignments to remain are the source of long-term degenerating processes like arthritis and disc disease. Chiropractic offers the safest most direct method of restoring a strong healthy neck. If you or someone you know suffers from neck or arm symptoms tell them about chiropractic.

FUNNY BONE: Ambition is a poor excuse for not having enough sense to be lazy.@@ We are all here on earth to help others; what on earth the others are here for I don't know.
Visit our web site: keefeclinic.com-ebook/keefeclinic.com
Understanding nutritional therapy

Most people today realize the importance of natural laws like proper diet and nutrition when it comes to good health. It was only about 45 to 50 years ago that even medical doctors weren’t so sure about that. I remember watching a debate between a cardiologist and a lay nutritional expert arguing about the importance of nutrition for healthy hearts. The cardiologist actually stated that foods are only needed for energy and there was no other nutritional benefit from eating foods. He totally dismissed the importance of vitamins and minerals and he was a doctor. So the fact that laypeople might have confusion about diet is understandable. There are 2 components about nutrition that I would like to speak to. One of those is diet and matching the proper ratio of fats,carbs and proteins to particular genetic makeup.

In some ways we’re all the same but in other ways we are different. Our genetic code determines how we utilize certain nutrients. Our body shapes will require different ratios of fats carbohydrates and proteins. So the foundation of proper nutrition is eating the right diet for your particular body type.

Nutritional therapy, using supplements to balance body chemistry, is the focus of this discussion. Just like each different body type requires a different ratio of fats carbs and proteins each individual has slightly different demands for certain nutrition. That’s one reason why a multivitamin is completely unable to supply what a given person needs. There are even some studies that show negative effects from taking multivitamins. We only give multivitamins in very specific cases. Our main goal is determining what nutrients are needed in reestablishing a better chemical balance as well as health status.
Different things can impact on your demand for nutrients, stress, exercise or the lack of exercise, disease, toxicity and many other factors. As these factors change your nutritional needs will change and so nutritional therapy is a dynamic and ever-changing approach towards health. The idea that you can take 4 or 5 nutrients that are most commonly talked about in the news is not the way to maintain good health.

Working with nutritional needs since 1976 I have found that nutritional therapy is quite a dynamic endeavor and one might need to change their program on a regular basis as her body reaches higher levels of function and health. We might pick up a nutrient that you need that you might only take for a week or a month or maybe over a year. Our testing procedures allows us to not only determine what nutrients or nutritional formula that you need but we can also determine how long you need to take that.

Many people don’t realize the advancements in nutritional therapy over the past 40 some years. The fact is with today’s knowledge there are very few health issues that can’t be effectively addressed. Acid reflux, cardiovascular disease, different hormonal imbalances, reoccurring infections, allergies and blood sugar disorders are just a few of the conditions that can respond. But the process isn’t as simple as we find a few nutritional formulas and you take those and everything clears up. It’s more complicated than that, because there can be several layers of dysfunction with any given problem. For instance you might have a blood sugar problem that could be related to the liver issue and adrenal gland dysfunction and an immune system disorder. Each of those will need to be addressed and sometimes in a specific order to resolve the original blood sugar problem. So during the process of treating the presenting blood sugar problem is going to be a time when we need to focus on the liver, and then the adrenals and lastly the immune system. Each of those could require separate nutritional approaches at separate times. Sometimes the body can’t address everything at once. So through periodic testing and re-addressing the nutritional program that you’re on full healing can take place over time.

When we go about developing an overall strategy to help someone recover their health certain testing might be indicated. A complete physical exam, blood work and other laboratory testing as well as Vega testing. Vega testing is a biofeedback form of testing that allows us to plug into your computer, so to speak, and pick up important information concerning your nutritional needs. Individualizing nutritional programs produce the best results in the long run. Vega testing allows us to individualize an approach and also minimize the number of nutrients needed in an effective program.

The most important part of the supplemental program is taking the supplements on a regular enough basis to get well. Some people have expressed difficulty in swallowing pills. There are a few secrets in making this process easier. One of them is taking your supplements with food, if were talking about tablets putting a little bit of butter on the tablet allows it to go down easier. Capsules are the easiest to swallow if you have the right head position. Capsules will float on water where tablets tend to sink. So when you’re swallowing a capsule lean your head forward and if you’re swallowing a tablet lean your head back. It’s best not to combine capsules and tablets if you have any problems swallowing pills. I have found when you’re taking in water right before you swallow the pill breathe in through your nose. This will help close off the windpipe so supplements don’t try to go down the wrong way and will make swallowing pills much easier.
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

DIET: Popcorn: Good or Not so Good? Anthropologists have found evidence that popcorn was part of indigenous diets in Mexico and Peru around 5,000 years ago and the Southwest around 2,500 years ago. Manganese, magnesium, phosphorus, zinc, copper, vitamins B3 and B6 and potassium in popcorn help reduce your risk of heart attack, stroke and atherosclerosis. Fiber and polyphenols are two popcorn ingredients that help make it relatively healthy, as they help fight cancer, type 2 diabetes and many other diseases. While 90 percent of the corn grown in the U.S. is genetically engineered (GE), popcorn is not (yet). Air-popped and with only coconut oil and a little salt, it’s a nourishing snack. It’s the additives that make popcorn potentially toxic.
IN THE NEWS: A POTENTIAL LINK BETWEEN CELL PHONE USE AND CANCER – JUNE 5, 2016 Use of cell phones is so deeply associated with our lifestyle that we often forget that these devices were almost nonexistent a decade ago. Therefore, we don’t have much feedback on their potentially harmful effects on health. Cell phones emit radio frequency radiation in very close proximity with our heads and it is still not clear if that radiation may present health issues. Researchers from the National Toxicology Program (NTP) were asked by the US government to investigate this question. They published a preliminary report on their findings on the association between radio frequency exposure through cell phone use and cancer. They exposed groups of 90 rats, separated by gender, to 3 different doses of radio frequency radiations (1.5, 3 and 6 W/Kg) for 9 hours per day, for 2 years. They found a higher incidence of brain (glioblastoma) and heart (Schwannoma) tumours in male rodents exposed to the radiation. Note: Not all people have the same susceptibility, we can test you on your cell phone plus there are phone covers that protect from this type of radiation.

CONDITION OF THE WEEK: Great Britain’s Most Outspoken Cardiologist Sets the Record Straight on Saturated Fats Saturated fat and cholesterol have little to do with the development of heart disease. Data shows two-thirds of people admitted to hospitals with acute myocardial infarction have completely normal cholesterol levels. Fats can be harmful, but it’s important to be specific. Fats that contribute to heart disease are primarily trans fats and highly refined and/or heated polyunsaturated vegetable oils (PUFAs), which are high in damaged omega-6. For optimal health, seek to get 75 to 85 percent of your total calories as healthy fat, primarily monosaturated and saturated. Limit PUFAs to 10 percent and omega-6 fats to 5 percent. Note: Chiropractic care and nutritional therapy are the best approaches to prevent heart problems.

FUNNY BONE: People who think they know everything are a great annoyance to those of us who do. @@ I want my children to have all the things I couldn't afford. Then I want to move in with them. @@ The closest a person ever comes to perfection is when he fills out a job application form.@@ I’m writing my book in fifth person, so
every sentence starts out with: “I heard from this guy who told somebody …”
LINK:getting-your-vegetables/
Visit our web sites: keefeclinic.com&facebook.com/keefeclinic
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: Heartburn Can Be Easily Eliminated Without Hazardous, Habit Forming Drugs Proton pump inhibitors (PPIs) like Nexium, Prilosec and Prevacid are designed to treat a very limited range of severe problems, yet as many as 20 million Americans take them for mild to moderate heartburn. PPIs have been linked to a heightened risk for a number of serious health problems, including chronic kidney disease, pneumonia, osteoporosis, hip fractures, dementia, heart disease, and heart attacks. There are many ways to eliminate heartburn or acid reflux without drugs, starting with eating real food and probiotic-rich fermented foods. Helpful supplements that help treat the underlying cause are also reviewed.

WELLNESS: Fluoride, the Only Drug Intentionally Added to Your Tap Water Fluoride is an endocrine disrupting, neurotoxic, and bone-weakening substance that the Food and Drug Administration (FDA) defines as a drug when used to prevent disease, such as dental caries. FDA recently announced that marketing fluoride drops and tablets for cavity prevention violates federal law, because FDA has never approved these products as either safe or effective. With water fluoridation, similar doses of fluoride contained in prescription fluoride drops and tablets are added to each glass a toddler drinks, without medical supervision, and without informed consent. NOTE: ALWAYS DRINK FILTERED WATER.

CONDITION OF THE WEEK: MUSCLE PAIN that hangs on can be annoying. Be it from an accident, beginning an exercise program or just sleeping in the wrong position, adjustments and trigger point therapy can be quite effective. Sometimes you can have muscle problems due to toxicity and we offer several detox programs to help . Nutritional deficiencies can also play a part in muscle pain. Magnesium is the most deficient mineral in the American diet and many people need to supplement with magnesium. For muscle injuries Pain Relief Cream is extremely effective. Don’t forget to drink enough water because dehydration can lead to muscle problems: your water needs are based on your body weight. One half of your body weight in ounces is your flushing or cleansing dose, 80% of that is your minimum dose. For instance a 100 pound person, then 50 ounces is a cleansing dose and 40 ounces is the minimum dose per day in fluids.

FUNNY BONES: A tom cat and a tabby cat were courting on a back fence at night. The tom leaned over to the tabby with pent up passion and purred... "I'll die for you" The tabby gazed at him from under lowered eye lids and asked, "How many times?".@@ An older man, not in the best physical condition, asked the trainer in the gym, "I want to impress that beautiful girl. Which machine should I use?" The trainer replied..."Use the ATM machine outside the gym!"@@ We are all here on earth to help others; what on earth the others are here for I don't know. @@ The first time I sang in the church choir; two hundred people changed their religion.@@ I knew I was an unwanted baby when I saw that my bath toys were a toaster and a radio.- Joan Rivers
Visit our web site: keefeclinic.com&facebook.com/keefeclinic
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: Staying in the game after concussion tied to longer recovery Continuing to play with a concussion may delay athletes from eventually returning to their sport by doing further damage to the brain and lengthening recovery time, a small U.S. study suggests. Past research has shown that "the brain is likely vulnerable to further physiologic and metabolic changes right after an injury - whether that be from sustaining more impacts or even from continued physical exertion," said lead author Breton Asken, a neuropsychology graduate student in the University of Florida's Clinical Psychology doctoral program. Athletes who played football, soccer, basketball, swimming, diving, volleyball and other sports were included. "Our findings indicate that immediately engaging your medical staff if you suspect you have sustained a concussion will give you the best chance to return to your sport more quickly," Asken said. Side note: once you have a concussion it’s more likely you could have another one with lesser of an injury. The more concussions the more brain damage.
WELLNESS: Mumps Being Spread by and Among Vaccinated People Merck stands accused of falsifying data to artificially inflate the efficacy rating of their mumps vaccine. There is evidence to suggest the vaccine is, indeed, not working. At least 40 students at Harvard University have contracted mumps, and every single one has been vaccinated. Outbreaks in 2009 and 2006 also involved a majority of vaccinated people. The White House is seeking $2 billion to create a vaccine against the Zika virus, just like they did for bird flu, swine flu, SARS, and so many other false alarms that translate into drug company profits when taxpayer dollars fund the development of new vaccines.
CHIROPRACTIC: Natural healthcare can help you Studies show more people are searching for natural solutions for their health. In 1993 the American Medical Association is a survey of health habits of Americans and found that more Americans visited doctors are practiced natural healthcare and doctors practice drug therapy and surgery. Chiropractic care, homeopathic care, individualized diet, acupuncture, nutritional therapy, muscle therapy and exercise, whatever it takes to move your body from disease to health, dysfunction to realizing your genetic potentials. We will customize a program to fit your particular health needs, from low back pain and headaches to digestive problems and PMS. From ear infections in babies to arthritis or heart disease and the geriatric patient, we offer the latest and natural healthcare. TELL A FRIEND

FUNNY BONE: An avid duck hunter was in the market for a new bird dog. His search ended when he found a dog that could actually walk on water to retrieve a duck. Shocked by his find, he was sure none of his friends would ever believe him.
He decided to try to break the news to a friend of his, the eternal pessimist who refused to be impressed with anything. This, surely, would impress him. He invited him to hunt with him and his new dog. As they waited by the shore, a flock of ducks flew by. They fired, and a duck fell. The dog responded and jumped into the water. The dog, however, did not sink but instead walked across the water to retrieve the bird, never getting more than his paws wet. This continued all day long; each time a duck fell, the dog walked across the surface of the water to retrieve it. The pessimist watched carefully, saw everything, but did not say a single word. On the drive home the hunter asked his friend, "Did you notice anything unusual about my new dog?"
"I sure did," responded the pessimist. "He can't swim."
Visit our web site: keefeclinic.com-ebook/keefeclinic.com
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: DISEASE-FIGHTING FOODS You’ve probably noticed that a little bit of color— in your closet and on your plate— is a great way to make things more attractive and inviting. It’s true; colorful foods are enticing to both grown ups and kids. And colorful fruits and vegetables also help protect you from chronic diseases, such as heart disease, stroke, some cancers, diabetes and obesity. “Eating in Color” takes you through the entire color spectrum, providing recipes and ideas for adding more reds, oranges, yellows, greens, blues, indigos and violets, and blacks and tans to your meals. Here's a taste of what each color can specifically do to boost a woman’s health. http://www.foxnews.com/health/2016/03/24/colorful-foods-that-fight-disease-and-aging.html

WELLNESS: More Than Half of The American Diet is Ultra-Processed Junk Food An astonishing 60 percent of the food Americans eat is ULTRA-processed, and these foods account for 90 percent of the added sugar consumption in the U.S. About 2 percent of the calories in processed foods come from added sugars. By definition, unprocessed or minimally processed contain none. Ultra-processed foods get 21 percent of their calories from added sugars. The 2015 U.S. dietary guidelines recommend limiting sugar intake to a max of 10 percent of daily calories. Cutting down on ultra-processed foods is a simple way to reduce your added sugar intake.

CONDITION OF THE WEEK: INJURIES AND CAR ACCIDENTS The number one cause for disability in any Western country is due to automobile accidents. What is even more concerning is patients will have marked disability 10 to 20 years after an accident in which they didn’t feel they were hurt. With any accident, be it a car accident or slip and fall, you can misaligned vertebrae in your neck or back that if not corrected will lead to degenerate over time. You can misaligned other joints like elbow joints, wrist, knee or ankle that because of mechanical friction arthritis can develop. Chiropractic adjustments of the neck, back, arm or leg can restore normal joint function and prevent degenerative changes.

FUNNY BONE: Girls are like phones. We love to be held and talked to, but if you press the wrong button you'll be disconnected!@@ You love flowers, but you cut them. You love animals, but you eat them. You tell me you love me, so now I'm scared!@@ All you need is love. But a little chocolate now and then doesn't hurt.@@ If you hold a cat by the tail you learn things you cannot learn any other way.@@ My wife never gives up. She is so insistent that she entered the wrong password over and over again until she managed to convince the computer that she's right!
Linkgetting-your-vegetables:
Link: crohns-disease
Visit our web site: keefeclinic.com-facebook/keefeclinic.com
Heart disease

The cholesterol theory of heart disease has been disproven several years ago. The most common cause for heart is an inflammatory chemistry. Junk food, stress a diet out of balance can all lead to an inflammatory chemistry. Inflammation can lead to changes in the coronary arteries which lead to narrowing of the arteries which can lead to a heart attack or stroke. Ways of reducing inflammation in the body includes eating anti-inflammatory foods ( http://www.keefeclinic.com/wp/foods-that-reduce-inflammation/ ), fish oils are very beneficial, drinking alkaline water as well as alkaline producing foods. Antioxidants like pomegranate plus is very protective and Heart Health has herbs for circulation to the heart. I include the linked products for your consideration.

click to order

click to order

click to order
To your better health,
Dr. Keefe
If you have not set up your account at natural partners then go to npscript.com/Keefeclinic and set up an account. There is no charge to set up this account and they do not sell email addresses. Once the account is set up you can order any of their over 9000 products in the comfort of your own home and have them shipped to your door. Note: for period of time you can get free shipping, order today!
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

IN THE NEWS: Heartburn Can Be Easily Eliminated Without Hazardous, Habit Forming Drugs Proton pump inhibitors (PPIs) like Nexium, Prilosec and Prevacid are designed to treat a very limited range of severe problems, yet as many as 20 million Americans take them for mild to moderate heartburn. PPIs have been linked to a heightened risk for a number of serious health problems, including chronic kidney disease, pneumonia, osteoporosis, hip fractures, dementia, heart disease, and heart attacks. There are many ways to eliminate heartburn or acid reflux without drugs, starting with eating real food and probiotic-rich fermented foods. Helpful supplements that help treat the underlying cause are also reviewed.

WELLNESS: IS YOUR BODY A TOXIC DUMP FOR CORPORATIONS? More than 200 industrial chemicals, found in products like pesticides, jet fuel and flame retardants, are found in Americans’ blood and breast milk. Chemical companies spent $100,000 lobbying per member of Congress in 2015 to successfully block serious oversight. Toxins you’re exposed to while in your mother’s womb can end up impacting the health of your great-grandchildren through inherited epigenetic changes. Ask us about a detox program, body ecology is a foundation for good health.
CONDITION OF THE WEEK: HEART DISEASE is a condition that is best treated through natural methods. Studies show that most medical approaches don't extend life as much as reports would indicate. Balloons, stents and bypass surgery come with several complications. Chiropractic adjustments help balance the parasympathetic and sympathetic nervous system which is crucial to heart health. Targeted nutrients to the blood vessels that surround the heart can cause dramatic improvement in short periods of time. Following your body type diet with emphasis on anti-inflammatory foods and balancing the body's pH can take a surgical heart condition and turn it into a healthy heart in the matter of weeks.

FUNNY BONES: Laugh and the world laughs with you, snore and you sleep alone.@@ If you're naturally kind, you attract a lot of people you don't like.@@ We owe a lot to Thomas Edison - if it wasn't for him, we'd be watching television by candlelight.@@ The first time I sang in the church choir; two hundred people changed their religion.@@ When we talk to God, we're praying. When God talks to us, we're schizophrenic.@@ I hate housework! You make the beds, you do the dishes and six months later you have to start all over again.@@ When I eventually met Mr. Right I had no idea that his first name was Always.
LINK:measuring-stomach-phfunction-using-ph-paper
LINK:light-color-and-health
LINK: common-sense-approach-to-heart-problems/
Visit our web site: keefeclinic.com&facebook.com/keefeclinic
Courtesy of:
John H. Keefe III, D.C.
(918) 663-1111

CONDITION OF THE WEEK: CHILDREN’S HEALTH is in jeopardy in the United States. Chiropractic and nutritional therapy offer hope against the following statistics. 50% of US children have chronic disease/disorders, 21% developmental disabled, current rate of autism 1 in 28 boys. 2007 academic pediatrics, 43 to 54.1% of US children (32 million) have a chronic health condition. 2011 issues of pediatrics between 1997 and 2008,.. Autism, ADHD, or developmental disability,... rose to (over) 10 million, or more than 15% of all kids between the age of three and 17. 2011 archives of Gen. psychiatry.. US has the highest in the world lifetime rate of bipolar disorder at 4.4%. 2013 autism, ADHD, and other developmental disabilities equal 21% US children. kids-and-chiropractic-care/

DIET: DIET AND HEART FAILURE High consumption of sweetened drinks was linked to increased risk for heart failure in middle-aged and older men. Note that this finding reflected adjustment for a host of potential confounders including education, smoking status, physical activity, personal and family histories of disease, and other dietary factors. heart-problems-do-the-experts-know-what-they-are-doing/
IN THE NEWS: Government Officials Gut 1986 NCVIA and Betray Public Trust In 1986, Congress created a federal vaccine injury compensation program (VICP) to restrict civil lawsuits against vaccine manufacturers and negligent doctors when government mandated vaccines injure or kill. Two out of three vaccine injury claims are denied. In 2011, the U.S. Supreme Court completely shielded the pharmaceutical industry from all civil liability for injuries and deaths caused by FDA licensed vaccines, and the VICP has been systematically gutted. There has been no legal accountability for any corporation or individual who develops, licenses, recommends, promotes, administers, or mandates vaccines that injure and kill Americans.

FUNNY BONE: Nature abhors a vacuum, but not as much as a cat does.@@ Cats are smarter than dogs. You can’t get eight cats to pull a sled through snow.@@ A duck walks into a drugstore and asked for a tube of Chapstick. The cashier says to the duck, "that'll be a 1.49." The duck replies, "put it on my bill."
Link:/are-vaccines-safe-and-effective
LINK:flu-vaccine
LINK:kids-and-chiropractic-care
Visit our web sites: keefeclinic.com&facebook.com/keefeclinic.com
Common sense approach to heart problems
Do the “experts” know what they’re doing?

Most people realize that heart disease is not due to a drug deficiency or lack of preventive surgeries. Heart disease is both a lifestyle and diet problem. The most current research points to inflammation, not cholesterol, as the cause for most heart disease. But based on current medical procedures this information is totally ignored. Probably the biggest reason that this information is ignored is that pharmaceutical companies drive most medical research and thus medical procedures. Pharmaceutical companies are multibillion-dollar enterprises that primarily fund and direct medical practice. Pharmaceutical companies are looking after their bottom line not necessarily your health.

The cholesterol theory of heart disease has failed to be established by major research. In fact it has been said that every cholesterol study for the past 50 years has failed. The Framingham study is the longest-running study on heart disease and diet. It has found that over the age of 45 people who have a higher cholesterol level outlive the people who have a lower cholesterol level. You might want to read that statement one more time. Cholesterol is a bystander in the coronary heart disease epidemic. Inflammation and other factors damage the lining of the blood vessel. White blood cells sensing the damage enter in to the damaged area and pull in cholesterol, calcium and laydown fibrin in an attempt to heal the damage. Because the factors that are driving the inflammation continue the corresponding plaque builds up over time.

Within the inflammation soup you have chlorine, fluoride, methane gas from the intestines and acidic byproducts from an improper diet that make up the insult to the blood vessels. Also hormones like insulin are pro-inflammatory and thus too much junk food and refined carbohydrates play a role in the inflammation process.

Medical approaches have been shown through research to be very ineffective at addressing coronary heart disease. Yes they can reduce cholesterol numbers with drugs but as the Framingham study indicates that just increases the patient’s risk of death. Let’s just cover some of the common medical procedures. First we’ll talk about balloons and stents: in 2003 the Mayo Clinic found that these procedures did not reduce complaints, did not prevent heart attacks, did not prolong life, did not reduce the frequency of heart attacks or deaths and the flattening of plaque in the vessel walls not only regularly lead to bleeding into the vessel walls but small lumps became detached and floated into the bloodstream causing acute heart attack. The bottom line is stents increased death rates.

Stents cause higher death rate
New England Journal of Medicine, Cindy L Grines, M.D. Beaumont cardiologist
Worldwide study involve 900 patients at 62 centers. All patients in the study were treated within 12 hours of the heart attack. A higher death rate (2.4% stent and angioplasty versus 2.7% angioplasty) they also unexpectedly experienced decreased blood flow from stent placements. Dr. Grines says "the higher mortality rate and decrease in blood flow makes it necessary for additional research to be conducted before routine stenting can be recommended as a standard of care."
It appears no one is studying the research.
Although catheterization and stents have been claimed to be life-saving measures, they do not prevent heart attacks or save lives. This intervention is accompanied by a great placebo effect. Conclusions.... We could not demonstrate a long-term benefit of an early invasive strategy in reducing death or MI (invasive versus conservative treatment in unstable coronary syndromes ICTUS (invasive versus conservative treatment of unstable coronary syndromes)

Heart bypass not effective long-term
The average death rate for bypass surgery is greater than the average death rate of heart patients treated without surgery. Heart disease progresses faster in those who have had invasive surgery and in those treated non-surgically. Heart surgery kills thousands of patients per year and debilitate thousands more.

Benefits of taking aspirin each day
10 to 15% drop in coronary heart disease (in theory)
33 to 46 fewer deaths per 100,000
Adverse effects of aspirin
Death rate:
37% increase in gastrointestinal bleeding (68-117 per 100,000)
32%-38% increase hemorrhagic stroke (8-10 per 100,000)
Aileen Clark, professor of public health and research director at Warwick medical school, said, "It is clear that there is an incredible fine balance between the possible benefits and risk of intervention. We need to be extremely careful about over promoting aspirin intervention without having 1st fully understood these negative side effects."
NSAIDs may cause 20% of all heart failures and cause high blood pressure
(NSAIDs) caused a greater than 10 fold increase in the risk and congestive heart failure (CHF) archives of internal medicine June 2000; 160:777-784

The study followed more than 80,000 women between the age of 31 and 50 years who are initially hypertensive-free. 2 years later: women who use in NSAIDs 22 days or more per month, the risk of high blood pressure increased some 86%.
Sudden death after radiofrequency ablation of the atrioventricular node in patients with atrial fibrillation Journal of American College of cardiology. 2002; 40 (1): 105-110. DOI: 10.1 thousand 16/S0735-1097 (02) 01927-7
Results of 334 consecutive patients with AF who underwent AV node ablation, 9 had sudden heart attacks after the ablation. Conclusions sudden-death likely to possibly related to catheter ablation occurred in 7 of 334 patients (2.1%). Risk of sudden death is highest within 2 days after the procedure.
Rational approaches to preventing and treating coronary heart disease.

Chiropractic
Chiropractic adjustments help balance the sympathetic and parasympathetic nervous systems.
Multiple studies show people with ischemic heart disease have a decreased parasympathetic activity by over 30%. Over 80% of ischemic events are preceded by a dramatic reduction of parasympathetic activity.

Diet
The important thing about diet is that there are different body types that require different eating programs. Studies show the global use of the Heart Association diet has failed in every country that it has been tested in. So the first thing we need to do is determine your body type and get you eating the correct ratio of fats, carbohydrates and protein for your genetic makeup. Next we need to make sure were maximizing the reduction of inflammation. Besides eating the right diet there are key foods like salmon and tuna, certain vegetables and fruits that are high in antioxidants like pomegranate and blueberries. Eating properly reduces the inflammation that leads to heart disease. Along with diet targeted nutrition to support the heart and healthy blood vessels is crucial to recovery.

PH is important when it comes to inflammation and most people who have inflammatory issues are usually very acidic. Again diet can control pH and drinking alkaline water can be beneficial. As we talk about water we need to talk about a whole host of chemicals in the water that can damage your cardiovascular system. The chlorine/chloramine, fluoride can damage the arteries. Studies have shown trace amounts of pharmaceutical drugs in city water supplies that need to be filtered out. Drink clean water, dehydration can lead to inflammation. The formula for water intake is that your cleansing dose is half your body weight in ounces and your minimum dose is 80% of that. I hundred pound person should drink between 40 and 50 ounces of fluids every day.

Exercise
30 minutes of exercise 3+ times a week can improve heart function and reduce stress hormones that can lead to ischemic reactions to the heart and increase blood pressure. Intense exercise can be a problem if you don’t eat a large amount of antioxidants because intense aerobic exercises can increase the oxygen level of your blood to a point of inflammation because oxygen is acidic. So too much intense exercise can actually have a negative effect on your heart health depending on your antioxidant intake.

Relaxation
Meditation and/or prayer have been shown to extend life and improve health recovery when practiced on a regular basis. Music, nature walks, family time are just a few of the things that can help the stress the nervous system and thus the body.

Detoxification
Heavy metals, certain chemicals in our environment as well as waste products from normal metabolism can a burden to the body and contribute to heart disease. Fasting, detox diets and supplements targeted at the detox pathways can be important for health recovery.
Most people who have symptoms of heart disease and follow the above program would notice improvement of symptoms within a few days to a couple of weeks and be totally symptom-free and out of trouble within a months’ time. This represents a rational approach to eliminating heart disease over the long-term.




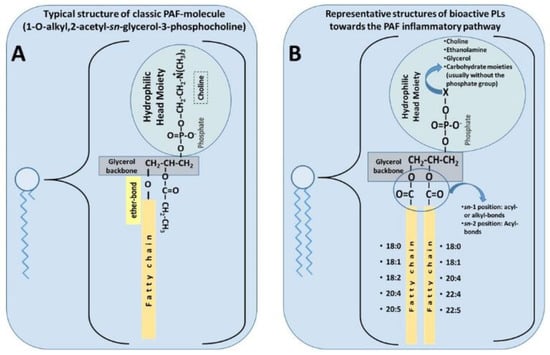
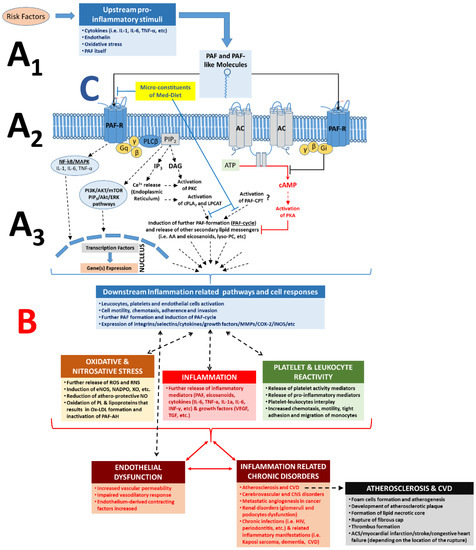
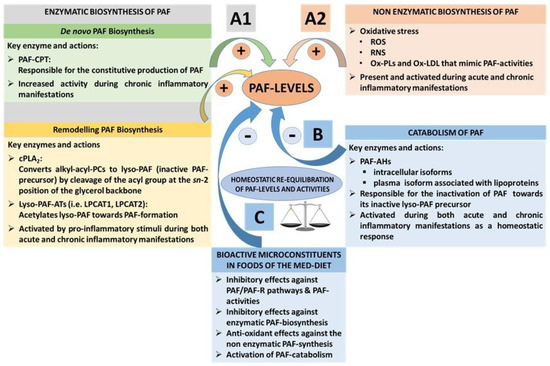
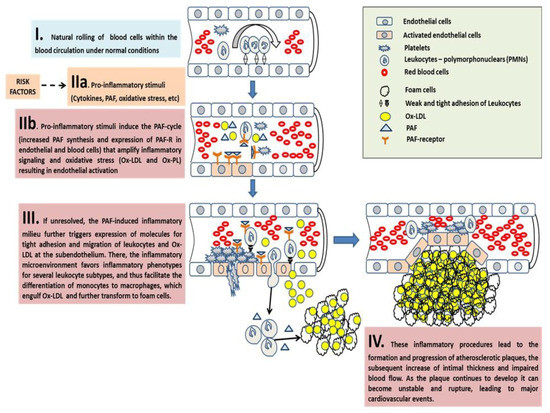


 This finding was particularly welcome, Folts notes, because most heart attacks occur in the early morning, sometimes just as an individual is getting up. His group's data now suggest that even if the juice had been drunk the day before, it would still confer some protection.
This finding was particularly welcome, Folts notes, because most heart attacks occur in the early morning, sometimes just as an individual is getting up. His group's data now suggest that even if the juice had been drunk the day before, it would still confer some protection.